Key Points
Childhood HL survivors are at elevated risk for neurocognitive impairment due to modifiable factors such as exercise and smoking.
Abstract
Long-term survivors of childhood Hodgkin lymphoma (HL) experience a high burden of chronic health morbidities. Correlates of neurocognitive and psychosocial morbidity have not been well established. A total of 1760 survivors of HL (mean ± SD age, 37.5 ± 6.0 years; time since diagnosis, 23.6 ± 4.7 years; 52.1% female) and 3180 siblings (mean age, 33.2 ± 8.5 years; 54.5% female) completed cross-sectional surveys assessing neurocognitive function, emotional distress, quality of life, social attainment, smoking, and physical activity. Treatment exposures were abstracted from medical records. Chronic health conditions were graded according to the National Cancer Institute’s Common Terminology Criteria for Adverse Events version 4.3 (1 = mild, 2 = moderate, 3 = severe/disabling, and 4 = life-threatening). Multivariable analyses, adjusted for age, sex, and race, estimated relative risk (RR) of impairment in survivors vs siblings and, among survivors, risk of impairment associated with demographic, clinical, treatment, and grade 2 or higher chronic health conditions. Compared with siblings, survivors had significantly higher risk (all, P < .05) of neurocognitive impairment (eg, memory, 8.1% vs 5.7%), anxiety (7.0% vs 5.4%), depression (9.1% vs 7%), unemployment (9.6% vs 4.4%), and impaired physical/mental quality of life (eg, physical function, 11.2% vs 3.0%). Smoking was associated with a higher risk of impairment in task efficiency (RR, 1.56; 95% confidence interval [CI], 1.02-2.39), emotional regulation (RR, 1.84; 95% CI, 1.35-2.49), anxiety (RR, 2.43; 95% CI, 1.51-3.93), and depression (RR, 2.73; 95% CI, 1.85-4.04). Meeting the exercise guidelines of the Centers for Disease Control and Prevention was associated with a lower risk of impairment in task efficiency (RR, 0.70; 95% CI, 0.52-0.95), organization (RR, 0.60; 95% CI, 0.45-0.80), depression (RR, 0.66; 95% CI, 0.48-0.92), and multiple quality of life domains. Cardiovascular and neurologic conditions were associated with impairment in nearly all domains. Survivors of HL are at elevated risk for neurocognitive and psychosocial impairment, and risk is associated with modifiable factors that provide targets for interventions to improve long-term functional outcomes.
Introduction
Hodgkin lymphoma (HL) is the most commonly diagnosed cancer in adolescence and has long benefited from high 5-year survival rates (87% in 19791), in part due to its radiosensitivity. This has resulted in a growing population of aging survivors who are at continued risk across the life span for treatment-associated morbidity and premature mortality.2-5 In the United States, there are >50 000 long-term survivors of childhood HL, many of whom are at risk of therapy-related cardiopulmonary and endocrine dysfunction, which increases their risk for neurocognitive and psychosocial impairments.2,6-8 However, the prevalence and correlates of neurocognitive impairment, emotional distress, diminished quality of life, and poor social attainment in adult survivors of childhood HL have not yet been comprehensively described.
The current study aimed to characterize neurocognitive, emotional distress, quality of life, and social attainment impairments in a well-characterized cohort of HL survivors relative to their sibling peers. We also aimed to identify demographic, clinical, treatment, behavioral, and chronic health factors associated with these impairments, hypothesizing that HL survivors would have more neurocognitive and psychosocial impairments compared with sibling peers. We also hypothesized that chronic health conditions would contribute to neurocognitive impairment and emotional distress and mediate associations between treatment exposures and neurocognitive impairment.
Methods
The Childhood Cancer Survivor Study
This cross-sectional study included survivors of childhood/adolescent HL enrolled in the Childhood Cancer Survivor Study (CCSS), a multi-institutional cohort of survivors of childhood/adolescent cancers diagnosed at ≤21 years of age between January 1970 and December 1999 who are ≥5 years from diagnosis.9,10 This analysis included all siblings enrolled in the CCSS as a comparator group. Siblings were the nearest-age sibling of a random sample of survivors from the larger CCSS cohort. The CCSS was approved by the institutional review boards at each of the 31 participating institutions, and all participants provided informed consent. For this analysis, survivors and siblings were ≥18 years old at the time of assessment and had no conditions predisposing them to cognitive/physical impairments not related to cancer or treatment (eg, trisomy 21 and traumatic head injury).
Neurocognitive and psychosocial measures
Neurocognitive functioning was self-reported using the Neurocognitive Questionnaire,11 which was developed and validated for use in childhood cancer survivors. Four domains are assessed: task efficiency (eg, processing speed, attention, and persistence), emotional regulation (control and expression of emotions), organization (of environment and plans), and memory (eg, immediate, short term, and long term). Neurocognitive impairment in each domain was defined as a T score ≥90th percentile based on a sample of adult-aged noncancer community residents.12
Emotional distress was assessed with the Brief Symptom Inventory-18, which has been validated in cancer survivors.13 Analyses excluded participants who completed this inventory via proxy (eg, parent). Scores were referenced to national normative data to generate age-adjusted T scores for domains of anxiety and depression, in which impairment was defined as a T score ≥90th percentile of population-based normative data.
The Medical Outcomes Short Form-36 assessed health-related quality of life.14 Eight specific domains of this form were used: general health, physical function, physical role function, physical role limitation, pain, emotional role limitation, vitality, and social functioning. Scores were referenced to national normative data to generate age-adjusted T scores for each of these domains, and impairment was defined as a T score <40 (mean ± SD, 50 ± 10).
Participants self-reported various metrics of social attainment, including education, income, employment, and independent living. Analysis of these measures was restricted to those ≥25 years old at survey completion. Education (college degree or higher vs some college education and lower) and personal income (less than $20 000 vs $20 000 and above per year15) were dichotomized. Employment was used at 3 levels: full-time, part-time, and unemployed. Living independently included those who endorsed living “alone”; with “spouse,” “roommate,” “children,” or “friends”; “in dormitory”; or “in military.”
Demographic, treatment, behavioral, and chronic condition risk factors
Participants completed questionnaires on demographic and behavioral factors such age, race, physical activity, and smoking (current smoker [≥100 cigarettes in his or her lifetime and smoking within the last month], former smoker [≥100 cigarettes in his or her lifetime and no smoking within the last month], and nonsmoker). Participants completed 6 questions on type, intensity, and frequency of physical activity from the Behavioral Risk Factor Surveillance System as well as 1 question about physical activity over the past month.16,17 Physical activity was categorized (yes/no) as meeting the physical activity guidelines of the Centers for Disease Control and Prevention (CDC) (150 minutes of moderate or 75 minutes of vigorous intensity activity per week). Body mass index was calculated by using self-reported height and weight.
Diagnosis and treatment history (within first 5 years of diagnosis) were abstracted from medical records at the participant’s treating institution (supplemental Figure 2, available on the Blood Web site). Chemotherapy and radiation treatments were operationalized dichotomously (yes/no) in addition to treatment intensity groups based on modern risk-adapted therapy as previously defined by Oeffinger et al.18 Risk factors included subsequent malignant neoplasms or recurrence of the primary cancer occurring ≥5 years from diagnosis, which were confirmed by review of pathology, medical records, and/or death certificates. In this study, late recurrence or subsequent malignant neoplasms were combined into one variable (yes/no) as a surrogate for additional cancer treatments not captured in CCSS.
At enrollment and each follow-up evaluation, participants completed questions regarding physical health conditions with age at onset (supplemental Figure 2). Health conditions that occurred up to the date of the follow-up questionnaire were included. Conditions were graded according to a modified version of the National Cancer Institute’s Common Terminology Criteria for Adverse Events version 4.3 (1 = mild, 2 = moderate, 3 = severe/disabling, and 4 = life-threatening).19 We considered whether participants had any grade 2 or higher chronic conditions (using maximum grades) for 4 different organ systems (cardiovascular, respiratory, endocrine, and neurologic) (supplemental Table 1). It is worth noting that neurologic conditions included grade 1 sensory neuropathy as all sensory neuropathy conditions in CCSS are considered grade 1.
Statistical analysis
Survivors were compared with siblings on demographic characteristics by using generalized estimating equations (GEE) accounting for potential within-family correlations. Frequency of impairment in each neurocognitive, emotional distress, and quality of life domain was calculated, and risk of impairment in survivors compared with siblings was estimated by using GEE with a Poisson distribution and log link. Among the HL survivors, multivariable Poisson regression for binary variables with the robust Huber-type sandwich standard error, estimated risk of impairment in each domain associated with demographic, lifestyle, and individual treatment factors and adjusted for time since diagnosis. This model was repeated replacing individual treatment factors with HL-specific treatment intensity groups.18 Participants who were missing treatment data were omitted from treatment-related analyses (N = 173). Chest and neck radiation were highly correlated, and therefore chest radiation was used as a surrogate for either. Because we hypothesized that chronic health conditions would be on the causal pathway between treatment and neurocognitive, emotional, and quality of life impairments, separate robust Poisson models excluding treatment exposures examined the risk of impairment associated with having a grade 2 or higher condition (vs grade <2) in each organ system. Models were mutually adjusted for all 4 organ system categories. The risk of impairment was examined for specific health conditions in which a meaningful number of events were observed, including hypertension, stroke, hypothyroidism, and sensory neuropathy.
Path analysis examined whether the associations between treatment factors and neurocognitive impairment were mediated by chronic health conditions. A hypothesized a priori model was generated based on existing literature and findings of our primary analysis. Model building was an iterative process of adding clinically meaningful paths and removing nonsignificant paths until the best model-fitting criteria were achieved; a good fitting model includes a comparative fit index ≥0.95 and the root mean square error of approximation <0.05.20
GEE with the Poisson distribution and robust variance estimated the risk of worse social attainment in survivors than in siblings adjusted for age, sex, and race/ethnicity. Separate models estimated the risk of each of the following: low income, low education, and dependent living associated with neurocognitive impairment and emotional distress in one model and quality of life impairments in a second model. Multinomial regression estimated the risk of unemployment or part-time employment (relative to full-time) in a similar manner.
All models were adjusted for age (continuous), sex, and race. All statistical testing was two-sided and considered statistically significant at P < .05. All analyses were completed by using SAS 9.4 (SAS Institute, Inc, Cary, NC) or MPLUS version 7.11. Although there are a large number of domains measured here, they each represent distinct constructs, and therefore no adjustment for multiple testing was done. Borderline statistically significant associations, however, should be interpreted cautiously.
Results
Of the 2710 HL survivors and 5037 siblings who were eligible and contacted for the second follow-up survey (which included the variables of interest), 1781 and 3196, respectively, completed the survey and were eligible for this analysis (supplemental Figure 1). Survivor participants did not differ in age from nonparticipants but were more likely to be female and treated with radiotherapy (supplemental Table 2). Survivors had a median time from diagnosis of 23 years and a median age of 37 years, which was significantly older than siblings (median age, 33 years) (Table 1).
Neurocognitive impairment
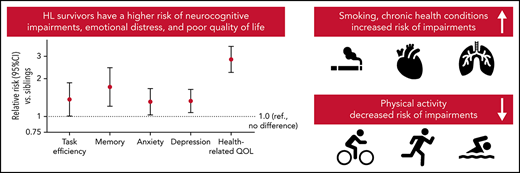
Survivors were statistically significantly more likely to be impaired after adjusting for age, sex, and race/ethnicity on neurocognitive domains compared with siblings, with relative risks (RRs) ranging from 1.32 for organizational impairment (95% confidence interval [CI], 1.01-1.73) to 1.72 for memory impairment (95% CI, 1.21-2.44) (Figure 1; supplemental Table 3). Among survivors, current and former smokers were at higher risk of impairments in task efficiency, emotional regulation, and memory (Table 2). Meeting CDC recommendations for exercise was associated with a lower risk of impairment in task efficiency (RR, 0.69; 95% CI, 0.51-0.93) and organization (RR, 0.59; 95% CI, 0.44-0.78). Obese survivors had a higher risk of task efficiency (RR, 1.64; 95% CI, 1.26-2.13) and memory impairments (RR, 1.61; 95% CI, 1.04-2.47). No statistically significant associations were found between treatment exposures and neurocognitive impairments, except for a positive association of anthracyclines on emotional regulation (RR, 0.54; 95% CI, 0.37-0.79) and corticosteroids on organization (RR, 1.57; 95% CI, 1.02-2.42). However, there was no correlation between anthracycline dose and neurocognitive impairment among survivors treated without chest radiation (n = 155) (supplemental Table 4). Treatment intensity was not significantly associated with neurocognitive impairment (supplemental Table 5). Having a late relapse (>5 years postdiagnosis) or a subsequent malignancy was associated with a higher risk of impairment on task efficiency (RR, 1.59; 95% CI, 1.13-2.24).
Risk of impairment in survivors compared with siblings. The figure includes the RR of impairment in each domain (and 95% CI) in survivors compared with siblings adjusted for age, sex, and race. The percentage impaired on each domain is also reported for survivors (in red) and siblings (in blue). The dotted line represents the expected rate of impairment in the general population.
Risk of impairment in survivors compared with siblings. The figure includes the RR of impairment in each domain (and 95% CI) in survivors compared with siblings adjusted for age, sex, and race. The percentage impaired on each domain is also reported for survivors (in red) and siblings (in blue). The dotted line represents the expected rate of impairment in the general population.
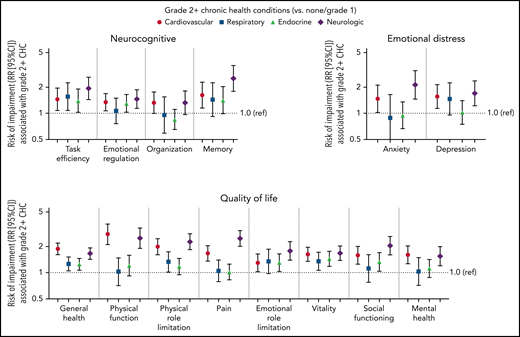
Grade 2 or higher cardiovascular or neurologic chronic health conditions were associated with higher risk of impairment in all 4 neurocognitive domains, and respiratory and endocrine conditions were associated with impaired task efficiency (RRs of 1.55 [95% CI, 1.07, 2.25] and 1.40 [95% CI, 1.03-1.91], respectively) (Figure 2). Hypertension was associated with impaired task efficiency (RR, 1.39; 95% CI, 0.97-2.00), emotional regulation (RR, 1.36; 95% CI, 1.03-1.79), and memory (RR, 1.61; 95% CI, 1.09-2.40) after adjustment for grade 2 or higher stroke and other cardiovascular conditions (supplemental Table 6). Stroke was associated with higher risk of impairment in task efficiency (RR, 3.24; 95% CI, 1.80-5.81) and memory (RR, 4.52; 95% CI, 2.45-8.33). Grade 2 or higher sensory neuropathy was associated with increased risk in all 4 neurocognitive domains.
The risk of impairment associated with chronic health conditions (CHCs). The RR of impairment (and 95% CI) associated with having a grade 2 or higher cardiovascular, respiratory, endocrine, or neurologic condition (compared with no grade 2 or higher conditions). Log-binomial models for each of the neurocognitive, emotional distress, and quality of life impairments included all 4 CHCs and were adjusted for current age, sex, and race.
The risk of impairment associated with chronic health conditions (CHCs). The RR of impairment (and 95% CI) associated with having a grade 2 or higher cardiovascular, respiratory, endocrine, or neurologic condition (compared with no grade 2 or higher conditions). Log-binomial models for each of the neurocognitive, emotional distress, and quality of life impairments included all 4 CHCs and were adjusted for current age, sex, and race.
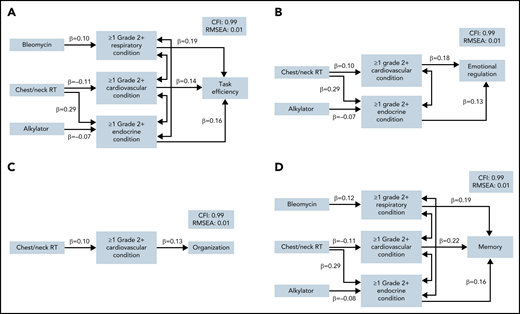
In mediation models, despite no direct effect of chest/neck radiation on neurocognitive impairment, there was a significant indirect effect of chest/neck radiation through cardiovascular and endocrine conditions on risk of impairment in task efficiency, emotional regulation, and memory (Figure 3; supplemental Table 7). These paths were associated with a 46% to 48% higher risk of impairment. Cardiovascular conditions also mediated the association between chest/neck radiation and organization, although not statistically significantly (P = .076). Lastly, there was a significant indirect effect of alkylators through endocrine conditions on memory impairment. Importantly, all associations between treatments and neurocognitive outcomes were completely mediated by chronic health conditions.
Structural paths for the mediation of treatment effects on neurocognitive function. Final path models are presented for each neurocognitive domain in which any indirect effects of treatment on neurocognitive function were noted (P < .10): (A) task efficiency, (B) emotional regulation, (C) organization, and (D) memory. Models are adjusted for current age, sex, smoking, and physical activity. Model fit indices are represented by the comparative fit index (CFI) and the root mean square error of approximation (RMSEA). Each model includes only significant paths and is labeled with a standardized β. Lines with double arrows indicate significant covariances between chronic health conditions.
Structural paths for the mediation of treatment effects on neurocognitive function. Final path models are presented for each neurocognitive domain in which any indirect effects of treatment on neurocognitive function were noted (P < .10): (A) task efficiency, (B) emotional regulation, (C) organization, and (D) memory. Models are adjusted for current age, sex, smoking, and physical activity. Model fit indices are represented by the comparative fit index (CFI) and the root mean square error of approximation (RMSEA). Each model includes only significant paths and is labeled with a standardized β. Lines with double arrows indicate significant covariances between chronic health conditions.
Emotional distress
Compared with siblings, HL survivors had a higher risk of clinically meaningful anxiety (RR, 1.31; 95% CI, 1.01-2.85) and depression (RR, 1.33; 95% CI, 1.08-1.64) (Figure 1; supplemental Table 3). Among survivors, current smoking was associated with a higher risk of impairment in anxiety and depression, whereas meeting CDC recommendations on physical activity was associated with a lower risk of depression (Table 2). Corticosteroids were associated with a higher risk of impairment in anxiety (RR, 1.99; 95% CI, 1.13-3.50) and depression (RR, 1.49; 95% CI, 0.91-2.44). Treatment regimens were not associated with emotional distress (supplemental Table 5).
Grade 2 or higher cardiovascular or neurologic conditions were associated with a higher risk of impairment in both domains (Figure 2). Sensory neuropathy was associated with a higher risk of anxiety (RR, 2.52; 95% CI, 1.71-3.69) and depression (RR, 2.18; 95% CI, 1.57-3.02) (supplemental Table 6).
Quality of life
Survivors of HL were at higher risk of impairment in all quality of life domains compared with siblings, except for pain (Figure 1; supplemental Table 3). The greatest discrepancy between survivors and siblings was the general health domain, in which 30.4% of survivors were impaired compared with 9.7% of siblings. Among survivors, smoking and having an obese body mass index were associated with a higher risk of impairment in each of the 8 domains, whereas physical activity was associated with a lower risk in each domain (Table 2). Alkylating agents were associated with a higher risk of impaired general health (RR, 1.30; 95% CI, 1.05-1.61). History of late relapse or subsequent malignancy was associated with a higher risk of impaired general health (RR, 1.24; 95% CI, 1.03-1.49), physical function (RR, 1.43; 95% CI, 1.04-1.96), and vitality (RR, 1.25; 95% CI, 1.00-1.55).
Grade 2 or higher cardiovascular and neurologic conditions were associated with a higher risk of impairment on all quality of life domains (Figure 2). Grade 2 or higher endocrine conditions were associated with a higher risk of impairment in vitality (RR, 1.39; 95% CI, 1.14-1.68) and social functioning (RR, 1.28; 95% CI, 1.01-1.62). Grade 2 or higher hypertension was associated with an increased risk of impairment in general health, physical function, physical role limitation, pain, vitality, and social functioning (supplemental Table 6), whereas grade 2 or higher hyperthyroidism was associated with impaired general health (RR, 1.25; 95% CI, 1.03-1.50).
Social attainment
Compared with siblings, survivors of HL were more likely to be unemployed (RR, 2.02; 95% CI, 1.68-2.42) or employed part-time (RR, 1.71; 95% CI, 1.31-2.23) and have a personal income below $20 000 (RR, 1.80; 95% CI, 1.35-2.40) (Figure 4). Task efficiency impairment was associated with a higher risk of low income (RR, 1.42; 95% CI, 1.08-1.87) and unemployment (RR, 3.41; 95% CI, 1.91-6.07) (Table 3). Memory impairment and depression were also associated with higher risk of unemployment (RRs of 1.89 [95% CI, 1.02-3.48] and 1.87 [95% CI, 1.01-3.46], respectively), whereas anxiety was associated with a lower risk of unemployment (RR, 0.37; 95% CI, 0.17-0.81). Separate models examining associations between impairment in quality of life domains showed that physical function and physical role limitation impairments were significantly associated with increased risk of low income and unemployment.
Risk of worse social attainment in survivors compared with siblings. The figure includes the RR of worse social attainment (and 95% CI) in survivors compared with siblings adjusted for age, sex, and race. The percentage of survivors and siblings are also reported for survivors (in red) and siblings (in blue).
Risk of worse social attainment in survivors compared with siblings. The figure includes the RR of worse social attainment (and 95% CI) in survivors compared with siblings adjusted for age, sex, and race. The percentage of survivors and siblings are also reported for survivors (in red) and siblings (in blue).
Discussion
As hypothesized, more survivors, compared with siblings, exhibited impairment in neurocognitive function, anxiety, depression, and quality of life, and were more likely to be unemployed and have a personal income less than $20 000. Importantly, at long-term follow-up, these impairments were not associated with prior treatment but rather with potentially modifiable risk factors such as exercise, smoking, and chronic health conditions. The effects of treatment on neurocognitive impairments were mediated by cardiovascular, respiratory, and endocrine conditions. These data highlight the added burden of poor quality of life as well as neurocognitive and emotional distress sequelae to the well-recognized physiological morbidity and premature mortality in long-term survivors of childhood HL. However, they also identify avenues for future intervention-based research designed to improve these long-term outcomes.
The risk of neurocognitive impairment among survivors of central nervous system (CNS) tumors or those treated with CNS-directed therapies such as cranial radiation has been well established. Recent research suggests that non–CNS-treated survivors are also at risk for neurocognitive impairment, which is potentially exacerbated by chronic health conditions associated with treatment.21,22 A pilot study of 62 long-term HL survivors also reported impairments in attention and memory that were associated with abnormal cardiopulmonary function.23 We extend these findings from the previous single-institution report, identifying survivors who are at increased risk of task efficiency and memory impairments that subsequently placed them at risk for unemployment. Unlike previous reports, our study was able to examine associations with individual treatments specific to HL and treatment intensity, showing no associations with neurocognitive impairment. The effects of treatments seemed to be completely mediated by respiratory and cardiovascular conditions. Specifically, hypertension and stroke, two medical conditions that are potentially preventable or clinically manageable, were associated with neurocognitive impairment. Survivors of HL experience cardiopulmonary sequelae such as hypertension and stroke at higher rates and earlier than their noncancer peers; therefore, these data support survivorship care well into adulthood to prevent and/or identify and manage chronic health conditions to mitigate the risk of neurocognitive impairments.
In addition, smoking and meeting CDC guidelines for physical activity were associated with neurocognitive function. Data among the larger CCSS cohort suggest that physical activity, especially consistent physical activity, is associated with better neurocognitive function.24,25 However, these studies included only survivors from the original CCSS cohort diagnosed between 1970 and 1986; our analyses include survivors diagnosed through 1999 who received risk-adapted multimodal therapy that decreased radiation exposures and increased anthracycline and alkylator doses.18,26 We also expanded on these studies by examining physical activity alongside individual treatments and treatment regimens specific to HL to inform 2 possible approaches to impairment mitigation: treatment modification and physical activity intervention. Our data show that physical activity, rather than treatment exposures, is an independent predictor of neurocognitive/psychosocial impairments in HL suggesting reduction in treatment intensity was less beneficial for neurocognitive/psychosocial sequelae in this population. Exercise interventions seem feasible in survivors of childhood cancer27 and have been effective in improving neurocognitive functioning among breast cancer survivors.28 Collectively, these data suggest that interventions such as exercise designed to improve cardiovascular, respiratory, and neurologic health may have a positive impact on neurocognitive functioning in HL survivors.
We addressed a gap in knowledge in previous reports of anxiety, depression, and quality of life impairments in survivors of childhood HL by using a comprehensive approach to identify demographic, lifestyle, and treatment exposures associated with these impairments.29-32 Previous studies failed to find associations between treatment intensity and quality of life or emotional distress.29,30 In the current report, we examined the impact of individual treatments and report associations between corticosteroids and anxiety/depression as well as impaired organization. Corticosteroids have been associated with increased anxiety and depression symptoms in children on active treatment of asthma and acute lymphoblastic leukemia as well as long-term adult survivors of childhood cancer.33,34 It is possible that corticosteroid treatment causes dysregulation and subsequent chronic stimulation of the hypothalamus-pituitary-adrenal axis. Dysregulation of this axis has been associated with endocrine system impairments, including insulin resistance, diabetes, and thyroid hormone regulation, as well as high levels of inflammation and oxidative stress, which have been implicated in cancer-related neurocognitive impairment.21,35,36
Similar to other studies of adolescent and young adult survivors, HL survivors were more likely to be unemployed or have an income less than $20 000 compared with their siblings.34,37,38 Impairment on task efficiency was associated with low income and unemployment, highlighting the potential economic impact of a neurocognitive intervention in this population. We reported no difference in education level, similar to a study of British HL survivors and a population-based US study of adolescent and young adult survivors.38,39 Diagnosis during adolescence may confer an advantage, as a higher proportion of HL survivors achieve a college degree compared with survivor groups diagnosed at younger ages who receive intensive CNS-directed therapies (eg, acute lymphoblastic leukemia).40 It is possible that many survivors achieved educational milestones but experience late-onset physical and neurocognitive morbidity that makes maintaining employment difficult.41 In support of this hypothesis, we found that impairment in task efficiency and memory was associated with unemployment. However, these data are cross-sectional, and survivors may experience significant neurocognitive morbidity that makes obtaining employment difficult. Future research on occupation type/duration may be important to understanding the relationship between neurocognitive function, employment, and education, and whether survivors of HL are more likely to be employed in low-skill jobs despite similar education.42
The treatment of HL has changed over the past several decades, including decreased use of chest radiation, limiting our ability to generalize these results to all survivors of childhood HL. However, an estimated 200 000 survivors of adult and childhood-onset HL are alive today who were exposed to similar treatments, including chest radiation, and to whom these data do apply.43 It is estimated that up to 40% of high-risk and relapsed patients will receive chest radiation on contemporary protocols.44,45 We attempted to classify survivors according to contemporary treatment regimens/intensity but observed no significant associations with neurocognitive or emotional distress impairments. In addition, we examined treatment agents individually, as the use of many is widespread across cancer types (eg, anthracyclines). Interestingly, we found positive effects of anthracyclines on various impairments, including emotional regulation, but there was no correlation between anthracycline dose and T scores among survivors treated without chest radiation (n = 155) (supplemental Table 4), suggesting that any protective effects may be due to the inverse correlation with chest radiation. We observed that sensory neuropathy was associated with an increased risk of impairment in all neurocognitive domains. This is particularly noteworthy given the recent migration from vinca alkaloids, known to be associated with peripheral neuropathy, toward the targeted agent brentuximab vedotin, which has been associated with very low rates of neuropathy.46,47 As the treatment landscape for HL changes, additional research is warranted to examine the impact of targeted agents and immunotherapies on neurocognitive function, emotional distress, and quality of life.
Our study has several strengths, including a large North American cohort of HL survivors, validated survey instruments, detailed treatment history, and extensive follow-up. However, there are several limitations, including the cross-sectional and retrospective nature of the surveys. Data on smoking, exercise, and neurocognitive/psychosocial impairments are cross-sectional, and therefore we cannot make conclusions regarding temporality (eg, smoking may be a coping mechanism for anxiety). Data for quantification of pack-years of smoking are not available; future research with detailed smoking history is needed to fully examine these associations. In addition, because of the self-reported nature of chronic health conditions, it is likely that these conditions are underreported, which may underestimate the associations between chronic health conditions and neurocognitive impairment. Unfortunately, objective neurocognitive testing is unavailable in this cohort, and this study was limited to self-report, with the participants endorsing these items likely experiencing overt symptoms. Although self-report and objective measures of neurocognitive function do not correlate highly, they measure separate but important aspects of neurocognitive functioning, and both are used in clinical assessment.48 Self-report of neurocognitive functioning informs on the participant’s ability to meet the neurocognitive expectations of their daily environment; objective measures provide a snapshot of a participant’s ability to perform under ideal controlled conditions. It is possible there are many more survivors than we report here who experience more subtle but clinically meaningful impairments, which, for example, would be identified on objective neurocognitive testing and not self-report measures. This may explain why our prevalence of neurocognitive impairments is lower than that of a pilot study of HL survivors that used objective neurocognitive testing.23 These findings are also limited by a primarily white sample and may not be generalizable to all HL populations. The associations reported here should be confirmed in a larger, more diverse, cohort of HL survivors with objective measures of neurocognitive function and chronic health conditions.
In summary, survivors of HL experience significant neurocognitive, emotional distress, and quality of life impairments relative to their peers. In this cohort, these impairments were more frequently associated with modifiable factors such as smoking, reduced physical activity, and chronic health conditions rather than cancer treatment exposures. These data suggest that many HL survivors are vulnerable to the effects of chronic health conditions and unhealthy lifestyle factors on their neurocognitive and psychosocial functioning, and the findings support additional survivorship care to detect and manage chronic health conditions and promote healthy behaviors to prevent or mitigate these impairments. Future research should collect longitudinal data and continue to monitor these domains over changing treatment paradigms but also focus on interventions to mitigate or prevent such impairments in long-term survivors. Interventions designed to increase positive health behaviors (eg, exercise) and improve cardiopulmonary function should be prioritized.
Acknowledgments
The authors thank the participants and the institutional investigators in the CCSS.
This work was supported in part by the National Institutes of Health, National Cancer Institute, grants U24 CA55727 (G.T.A., Y.Y., R.H., K.C.O., E.J.C., W.L., D.S., M.M.H., and K.R.K.), K00 CA222742 (A.M.W., L.L.R., and K.R.K.), and P30CA021765-42 (Y.Y., M.M.H., L.L.R., K.R.K., and G.T.A.; Principal Investigator, Charles Roberts). Support to St. Jude Children’s Research Hospital investigators was also provided by the American Lebanese-Syrian Associated Charities.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The funding organizations had no role in the design and conduct of the study; collection, management, analysis, or interpretation of the data; preparation, review, or approval of the manuscript; and decisions to submit the manuscript for publication.
Authorship
Contribution: A.M.W., N.S.P., M.J.E., Y.Y., T.G., E.J.C., M.M.H., L.L.R., G.T.A., and K.R.K. were responsible for the concept and design of the study; A.M.W., G.T.A., and L.L.R. acquired financial support; M.M.H., L.L.R., G.T.A., and K.R.K. were responsible for acquisition of study materials, participants, and data; A.M.W., M.X., M.J.E., R.H., W.L., K.C.O., S.M.S., D.S., and K.R.K. were responsible for analysis and interpretation of data; all authors drafted the manuscript, tables, and figures; and all authors approved the manuscript as submitted, and all are accountable for all aspects of the work.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Kevin R. Krull, Department of Epidemiology and Cancer Control, St. Jude Children’s Research Hospital, 262 Danny Thomas Place, MS 735, Memphis, TN 38105-3678; e-mail: kevin.krull@stjude.org.
All data are from the CCSS, a National Cancer Institute–sponsored resource designed to inform on the long-term effects of childhood cancers. Data are available to all interested investigators (ccss.stjude.org provides additional information).
The online version of this article contains a data supplement.
There is a Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.