Abstract
Introduction:
Ibrutinib (Ibr) is the first approved irreversible BTK inhibitor. Cardiovascular (CV) adverse events, include hypertension (HT) and atrial fibrillation (AF). Ventricular tachyarrhythmias and sudden death have been reported possibly due to off target kinase inhibition. Several randomised Phase III trials have reported low numbers of cardiac deaths, sudden/unexplained deaths, and/or ventricular arrhythmias with Ibr including 9 deaths in RESONATE-2 Trial, 11 unexplained or unwitnessed deaths in Alliance Trial A041202 (med age 71; Ibr (n=7 [4%]), Ibr+ritux (IR; 4 [2%]), one death in the IR arm of ECOG1912 Trial (mean age 56.7) and 3 unexplained plus 5 cardiac deaths with BR + Ibr in the HELIOS trial (med age 64).
Methods:
FLAIR, a phase III, multicentre, randomised, controlled, open, trial recruiting 771 pts with untreated CLL requiring therapy. Pts were ≤75 years, considered fit for FCR and were randomized to IR or FCR. Pts with inadequately controlled symptomatic cardiac failure or unstable angina were excluded. We conducted ad hoc evaluations of the potential association of sudden or cardiac deaths with prior medical history or therapies for cardiac disorders (AF, Ischaemic heart disease, MI or angina) and HT via univariate analyses. We used Fisher's Exact test and estimated relative risk (RR) and associated confidence intervals under a normal approximation.
Results:
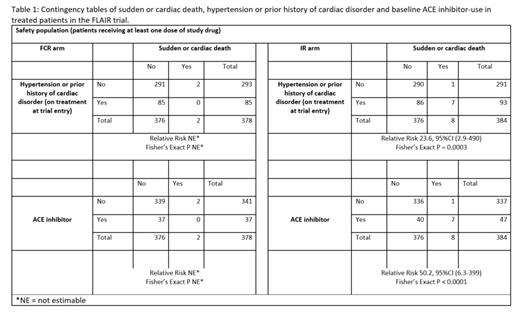
In FLAIR, 384 and 378 pts were treated with IR and FCR, respectively, median 52.7 months FU and treatment durations of 47.2 and 4.7 months. Median age 62. 84/384 (22%) IR pts had pre-existing HT and 17/384 (4%) a prior history of a cardiac disorder. 74/378 (20%) FCR pts had HT and 18/378 (5%) a cardiac disorder. To date 10 pts had a sudden or cardiac death: 2 (0.5%) FCR and 8 (2%) IR - median 33 mo (range: 13-48) from initiating IR to death. 9/10 pts were male aged 54 to 73 years at randomisation. 7/ 8 IR pts experiencing a sudden or cardiac death, had a prior history of HT and/or cardiac disorder (RR 23.6 vs pts with no HT/CV history, 95%CI [2.9-195]; Fisher's Exact P=0.0003; Table 1). Hypertensive cardiomyopathy and/or coronary artery disease was found at post mortem of all 3 IR pts having a PM. The risk of sudden or cardiac death with IR was 0.3% (1/291) in pts without pre-existing risk.
The number of pts taking anti-hypertensives at trial entry (some taking more than one class) was: ACE inhibitors (ACEi) 47 IR, 37 FCR; Angiotensin II receptor blockers (ARB's) 15 IR, 17 FCR; Ca channel blockers 30 IR, 34 FCR; B-blockers 24 IR, 20 FCR; Diuretics 13 IR, 16 FCR; and alpha blockers 7 IR, 5 FCR. Overall, 291/384 (76%) IR and 296/378 (78%) FCR were taking no HT or cardiac medications at trial entry.
The use of ACEi at study entry was associated with an increased risk of sudden or cardiac death with IR: 7/47 (15%) pts on ACEi at trial entry had a sudden or cardiac death compared to 1/336 (0.3%) not taking ACEi (RR 50.2, 95%CI [6.3-399]; P < 0.0001; Table 1). 2/7 pts had discontinued ACEi and switched to ARB's due to cough sometime prior to death. In contrast 0/37 FCR pts with a history of HTN/CV taking ACEi experienced a sudden or cardiac death. In the IR arm, none of the 46 pts receiving cardiac medication but not ACEi had a sudden or cardiac death suggesting that the risk was not simply a prior history of HT or cardiac disorder. 3/11 (27%) IR pts taking an ACEi plus a beta blocker at study entry had a sudden or cardiac death vs 4/32 (12.5%) pts on ACEi without a beta blocker.
Factors potentially confounding the interpretation: 1) analyses did not take into account severity of underlying HT/CV risk, as it is possible that pts on ACEi had more severe underlying CV disease than others; and 2) analyses did not adjust for changes in HT/CV medications during the course of the study. However, the lack of any such signal in pts with a prior cardiovascular/HT history on therapies other than ACEi suggests a potential association with the medication itself.
Conclusion:
Sudden or cardiac deaths seen on IR in FLAIR were observed predominantly among male pts with a prior history of HT or cardiac disease. The prior use of ACEi was correlated with the risk of sudden or cardiac death in IR pts. In contrast, pts treated with IR who were not on ACEi (regardless of medical history) had a very low rate of cardiac or sudden death. Whilst these findings need confirming in other Ibr trials we believe that caution should be taken when concurrently administering ibrutinib and ACE inhibitors in pts with a medical history of HT or CV disease.
Munir: F. Hoffmann-La Roche: Consultancy; Alexion: Honoraria. Bloor: Novartis: Honoraria; Kite, a Gilead Company: Honoraria. Broom: AbbVie: Honoraria; AstraZeneca: Honoraria; Janssen-Cilag Ltd: Honoraria; Takeda UK Ltd: Honoraria; Celgene Ltd: Honoraria; Gilead: Honoraria. Furtado: Abbvie: Other: Conference support. Morley: Roche: Membership on an entity's Board of Directors or advisory committees, Other: Conference support; AbbVie; Takeda: Other: Conference support; Janssen: Honoraria; Kite: Honoraria. Cwynarski: Atara: Consultancy; BMS/Celgene: Other; Celgene: Consultancy; Gilead: Consultancy, Speakers Bureau; Incyte: Consultancy, Speakers Bureau; Janssen: Consultancy, Other; Kite, a Gilead Company: Consultancy, Speakers Bureau; Roche: Consultancy, Other, Speakers Bureau; Takeda: Consultancy, Other, Speakers Bureau. Gatto: Roche: Consultancy. Paneesha: AbbVie: Honoraria; Bristol Myers Squibb: Honoraria; Gilead: Honoraria; Janssen: Honoraria; Roche: Honoraria; Celgene: Honoraria. Fox: F. Hoffmann-La Roche Ltd: Consultancy, Membership on an entity's Board of Directors or advisory committees. Howard: Roche: Current Employment. Cairns: Takeda: Research Funding; Amgen: Research Funding; Merck Sharpe and Dohme: Research Funding; Celgene / BMS: Other: travel support, Research Funding. Patten: NOVARTIS: Honoraria; ASTRA ZENECA: Honoraria; ABBVIE: Honoraria; JANSSEN: Honoraria; GILEAD SCIENCES: Honoraria, Research Funding; ROCHE: Research Funding. Hillmen: BeiGene: Honoraria; SOBI: Honoraria; AstraZeneca: Honoraria; Gilead: Research Funding; Roche: Research Funding; Pharmacyclics: Honoraria, Research Funding; AbbVie: Honoraria, Other: Travel, Accommodations, Expenses, Research Funding; Janssen: Honoraria, Other: Travel, Accommodations, Expenses, Research Funding.