Abstract
Recent studies have demonstrated that only 30% of patients referred for assessment of a possible bleeding tendency will eventually be diagnosed with a mild bleeding disorder (MBD) such as von Willebrand disease (VWD) or platelet function defect (PFD). Rather, most of these patients will be diagnosed with bleeding disorder of unknown cause (BDUC). There remains an important unmet need to define consensus regarding the clinical and laboratory criteria necessary for a formal BDUC diagnosis. Accumulating recent data suggest that BDUC is being diagnosed with increasing frequency. Objective assessment of bleeding phenotype using a standardized bleeding assessment tool (BAT) therefore represents a fundamental first step in the diagnosis of BDUC. Because BDUC is a diagnosis by exclusion, accurate quantification of bleeding phenotype is critical because this will be the primary determinant on which a diagnosis of BDUC is reached. Importantly, BAT scores suggest that patients with BDUC display bleeding phenotypes comparable to those seen in patients with VWD or PFD. Despite the prevalence of BDUC, diagnosis and management of these patients commonly pose significant clinical dilemmas. We consider these challenges in the context of a number of typical case studies, discuss the available evidence, and outline our approach to the management of these patients.
Introduction
Hematologists are frequently referred patients for assessment of a possible bleeding tendency. Studies of patients referred because of a personal or family history of bleeding have demonstrated that only 30% will ultimately be diagnosed with mild bleeding disorders (MBDs) such as von Willebrand disease (VWD) or a platelet function defect (PFD).1,2 Importantly, the most common final diagnosis in this group is the entity broadly called bleeding disorder of unknown cause (BDUC).1,3,4 Lack of standard diagnostic criteria for BDUC makes it is difficult to assess the true prevalence of this condition.3,5 However, patients with BDUC already account for more than 10% of registered patients in some hemophilia centers.6 Furthermore, cohort studies have reported that BDUC is being diagnosed with increasing frequency over recent years (Table 1), particularly in female patients presenting with heavy menstrual bleeding (HMB) and postpartum hemorrhage (PPH).1,4,6 Objective bleeding assessment tools (BATs) suggest that patients with BDUC display muco-cutaneous bleeding phenotypes comparable to those seen in patients with VWD or PFD.4,7-9 Nonetheless, physicians continue to face dilemmas with respect to the diagnosis and management of patients with BDUC. In this study, we consider these clinical challenges in the context of a number of typical case studies, discuss the available evidence, and outline our approach to managing these patients.
Diagnosis of BDUC
Case 1
A 33-year-old woman with persistent iron deficiency anemia is referred for investigation of a possible underlying bleeding disorder. She has a history of HMB dating back to menarche. Her periods typically last up to 10 days, requiring regular pad changing (every 1-2 hours) through the first 3 to 4 days. This HMB previously required a dilation and curettage. In addition, the patient has required 3 intravenous iron infusions over the last 5 years. More recently, the HMB has improved in response to a progesterone-only intrauterine contraceptive device. She also describes excessive bleeding after 2 wisdom teeth extractions that necessitated admission, packing, and resuturing. Finally, she has lifelong easy and extensive bruising. With respect to family history, her mother also had HMB, which ultimately led to a hysterectomy at 38 years of age. In terms of medications, the index case uses the episodic nonsteroidal anti-inflammatory drug (NSAID) ibuprofen for arthralgia and a selective serotonin reuptake inhibitor sertraline for chronic anxiety.
Discussion of case 1
BAT scores in BDUC diagnosis
Objective assessment of bleeding phenotype represents a fundamental first step in the assessment of our index case. This is best achieved using a standardized BAT.10 A number of different BAT iterations have been developed including the current BAT endorsed by the International Society on Thrombosis and Haemostasis (ISTH-BAT).10,11 For the ISTH BAT, a normal bleeding score (BS) is <4 in men and <6 in women.11,12 Previous studies have demonstrated that BATs are useful in assessment of patients being evaluated for potential MBDs.7,8,13-16 For example, Tosetto et al15 showed that a normal BS could reliably exclude the presence of an underlying MBD in unselected consecutive referrals (negative predictive value >99%). Similarly, BAT scores have also been shown to be effective for MBD screening in women presenting with HMB.7,14,16
More recent studies have examined the specific clinical utility of BATs in patients with BDUC.7,8,13,17 Accurate quantification of bleeding phenotype in these subjects is critical because this is ultimately the primary determinant on which a diagnosis of BDUC is reached. Five recent cohort studies examined BAT scores in consecutive patients referred to tertiary hospitals for hemostasis evaluation (Table 1).8,16-19 Overall, a high prevalence of BDUC was confirmed (up to 60% of patients) among these referrals. HMB, PPH, easy bruising, minor bleeding from wounds and oral cavity tooth extraction, and excessive bleeding at surgery were common. The studies consistently reported a female predominance in BDUC cases. For example, Relke et al7 reported that 98% of the patients were women and that HMB constituted the commonest type of bleeding. Overall, a wide range in BAT scores was observed (Table 1). Nevertheless, a significant number of patients with BDUC had high BS comparable to those seen in patients with VWD or PFD.7,8
Together, these data suggest that BAT scores are clinically useful in BDUC diagnosis. However, it is important to consider a number of inherent limitations. In children and adults who have been exposed to few previous hemostatic challenges, BS may be normal even in the presence of an underlying bleeding tendency.10 In addition, it is important that BS should be defined at time of first diagnosis. Falsely elevated scores may result if calculated retrospectively because BSs are impacted by prophylactic treatments given to cover elective procedures.20 Importantly, BATs have a low ability to discriminate between different causes of mild mucocutaneous bleeding (eg, VWD, PFD, or BDUC), which can only be differentiated after subsequent laboratory testing.4,7,8 Finally, the clinical utility of BATs in screening patients with mild bleeding disorders has been questioned.8,13 In particular, several studies have highlighted that the positive and negative predictive values of BS are strongly dependent on on the prevalence of bleeding disorders in the group undergoing testing. Consequently, some recent studies have reported that the negative predictive value of BATs may be as low as 66% in specific settings.8,13 Collectively, these findings underscore the importance of clinical gestalt in considering MBD referrals and emphasize the need for the involvement of experienced hemostasis physicians. Notwithstanding these limitations, a recent European Hematology Association consensus report recommended that the ISTH BAT be used to distinguish pathologic from trivial bleeding and to guide the need for further laboratory diagnostic workup in referred patients.21 In line with this approach, our practice is to calculate an ISTH-BAT score for all subjects with mucocutaneous bleeding referred for bleeding state workup. Based on her bleeding history, our index case had a significantly abnormal ISTH BAT score of 10 (positive BS ≥6 for women).
History and clinical examination in BDUC
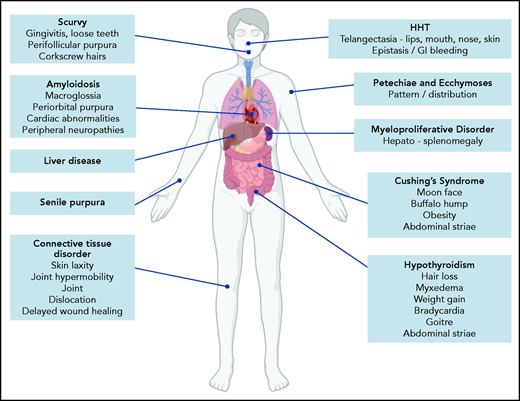
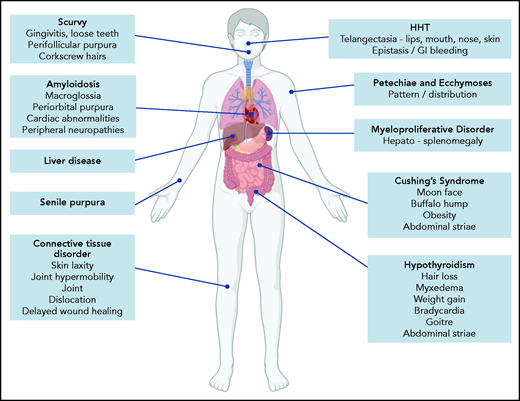
Because BDUC is a diagnosis by exclusion, a full medical history (beyond the BAT score) and clinical examination must be performed in patients referred with possible bleeding. Important aspects would include a family history of bleeding and to establish whether there is any consanguinity. A list of medications and health supplements is needed. Clinical examination may demonstrate petechiae or ecchymosis and should assess for signs of collagen vascular disorders (including skin laxity, joint hypermobility, joint dislocations, delayed wound healing; Figure 1).22 The pattern and distribution of bleeding observed may raise a number of possibilities (including self-inflicted or nonaccidental injury). In addition, signs associated with other inherited or acquired causes of mild to moderate bleeding disorders should be considered (Figure 1). Of note, our index case is using medications (NSAID and selective serotonin reuptake inhibitor) that may also contribute to her underlying bleeding phenotype. Her clinical examination was unremarkable.
Clinical features associated with inherited and acquired causes of mild to moderate mucocutaneous bleeding. HHT, hereditary hemorrhagic telangiectasia.
Clinical features associated with inherited and acquired causes of mild to moderate mucocutaneous bleeding. HHT, hereditary hemorrhagic telangiectasia.
Laboratory investigations in the diagnosis of BDUC
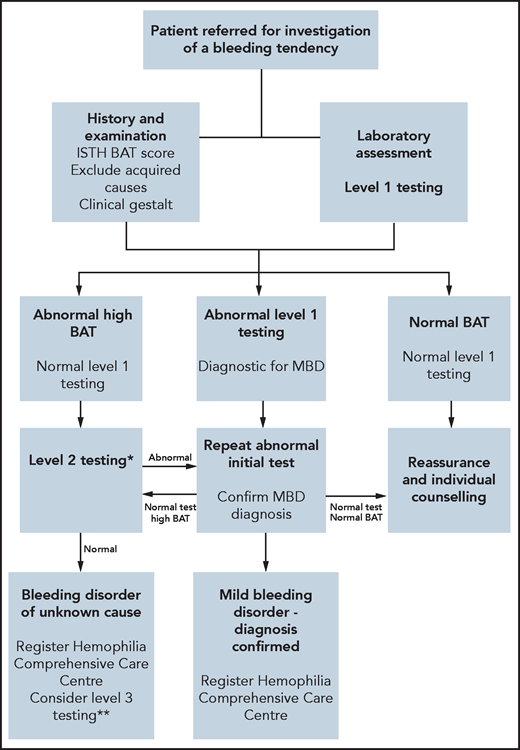
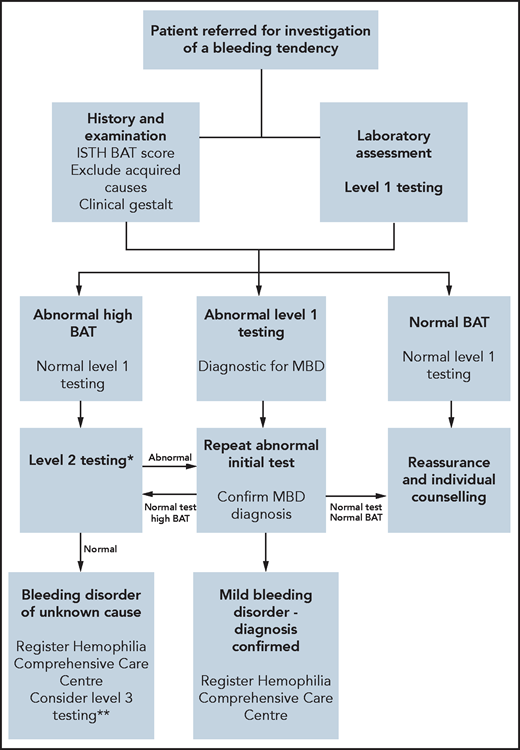
Because abnormal BS cannot differentiate between causes of mucocutaneous bleeding, laboratory investigations must be performed.21,23 For all patients with an abnormal BAT, we assess full blood count, ferritin, biochemistry profile, liver function tests, and C-reactive protein (CRP) (Table 2). Further specific tests may be indicated based on clinical history and examination. In patients with abnormal BATs, our first-line hemostatic testing includes a prothrombin time (PT), activated partial thromboplastin time (APTT), and fibrinogen level (Figure 2). Platelet morphology is assessed on a peripheral blood film. VWF (von Willebrand factor) antigen and function levels, together with factor VIII (FVIII), FIX, and FXI assays, are measured. ABO blood group is also determined. Previous studies have suggested that ABO blood group influences primary hemostasis through effects on both VWF and platelet function.24 Moreover, Mehic et al25 recently reported that blood group O was a risk factor for increased bleeding and bleeding severity in patients with BDUC, independent of VWF and FVIII levels. In view of the variability in plasma levels, VWF tests are repeated on 2 separate occasions several weeks apart.26 Finally, standard platelet aggregation light transmission aggregometry (LTA) with arachidonic acid, ADP, adrenaline, collagen, thromboxane B2 (TxB2) agonist and ristocetin, and platelet nucleotide testing are performed. In our index case, all these initial laboratory tests were within the normal range.
Proposed BDUC diagnostic algorithm. *Level 2 laboratory testing performed in patients with abnormal ISTH BAT scores but normal level 1 tests. **Level 3 laboratory testing is reserved for patients with normal level 2 testing but marked bleeding phenotypes (eg, IATH BAT >10 or recurrent anemia or strong family histories or planned procedures associated with major bleeding risks). Ideally, these level 3 tests should be performed in the context of a research study.
Proposed BDUC diagnostic algorithm. *Level 2 laboratory testing performed in patients with abnormal ISTH BAT scores but normal level 1 tests. **Level 3 laboratory testing is reserved for patients with normal level 2 testing but marked bleeding phenotypes (eg, IATH BAT >10 or recurrent anemia or strong family histories or planned procedures associated with major bleeding risks). Ideally, these level 3 tests should be performed in the context of a research study.
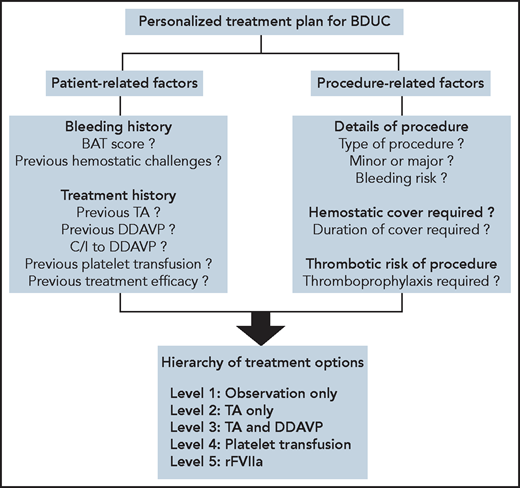
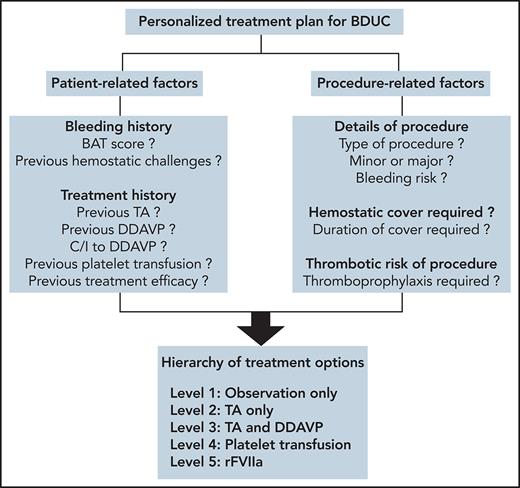
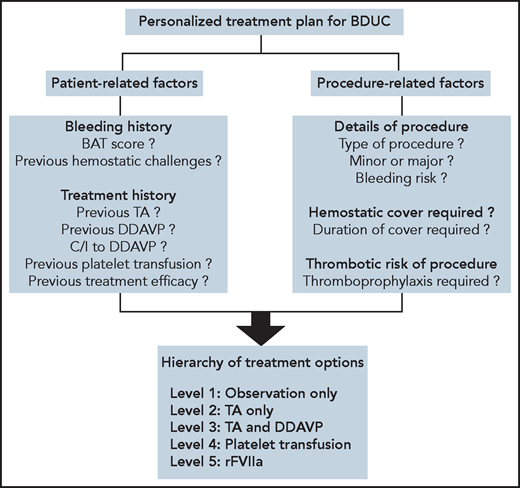
Considerations in developing personalized treatment plans for patients with BDUC. To develop a personalized treatment plan for a patient with BDUC, we first assess patient-related and procedure-related factors. A treatment plan for the specific procedure is then developed based on an ascending hierarchy of therapeutic options. In some patients with minimal objective evidence of previous bleeding, or in younger patients who have not undergone previous significant hemostatic challenges, with patient agreement we advocate an observation policy in the first instance. In these patients, TA and/or DDAVP are available on standby to manage any bleeding complications. For patients with previous procedure-related bleeding complications, we recommend TA alone or in combination with DDAVP before any significant future challenges. Platelet transfusions are used for patients with BDUC who develop bleeding complications despite therapy with TA and DDAVP. Finally, we only consider rFVIIa as a last-ditch option in patients with BDUC with ongoing active bleeding refractory to other treatment options. C/I, contraindication. All figures were created with BioRender.com.
Considerations in developing personalized treatment plans for patients with BDUC. To develop a personalized treatment plan for a patient with BDUC, we first assess patient-related and procedure-related factors. A treatment plan for the specific procedure is then developed based on an ascending hierarchy of therapeutic options. In some patients with minimal objective evidence of previous bleeding, or in younger patients who have not undergone previous significant hemostatic challenges, with patient agreement we advocate an observation policy in the first instance. In these patients, TA and/or DDAVP are available on standby to manage any bleeding complications. For patients with previous procedure-related bleeding complications, we recommend TA alone or in combination with DDAVP before any significant future challenges. Platelet transfusions are used for patients with BDUC who develop bleeding complications despite therapy with TA and DDAVP. Finally, we only consider rFVIIa as a last-ditch option in patients with BDUC with ongoing active bleeding refractory to other treatment options. C/I, contraindication. All figures were created with BioRender.com.
In cases where initial hemostasis testing is normal, we proceed to second-line laboratory testing (Figure 2). This includes measurement of individual clotting factor assays (FII, FV, FVII, FX, and FXIII; Table 2). When second-line testing demonstrates no abnormalities, some centers may perform additional laboratory testing to assess primary hemostasis under shear (eg, PFA-200),27 fibrinolytic pathway,28-33 and measure global hemostasis18,29,30,34,35 (Table 2). Importantly however, recent studies have highlighted that these third-line tests detect additional diagnostic abnormalities in only a minority of patients, and their clinical utility remains unclear.1 Moreover, performing a large array of tests clearly increases the possibility of identifying abnormalities by chance. Finally, high-throughput sequencing of genes associated with bleeding and platelet disorders has been assessed in patients with BDUC. However, in a study of 619 patients with unexplained bleeding, Downes et al36 identified abnormalities in only 3% of subjects studied.
For our index case, all first- and second-line laboratory tests were within the normal range. Consequently, based on her abnormal BS but normal laboratory testing, she was diagnosed with BDUC. This formal diagnosis has important future health care implications for the patient (Table 3).6,21 She was provided with a registration card and advised to minimize further use of NSAIDs. She was also provided with emergency contact information for her local Hemophilia Comprehensive Care (HCC) Center. In particular she was advised to contact the HCC directly should she require any elective procedures (including surgery or dental extractions) or for future pregnancies. Her primary care physician will monitor her iron status to determine success of the progesterone-only intrauterine contraceptive device to control her HMB.
Management of BDUC for major surgery
Case 2
A 45-year-old man registered with BDUC required a tonsillectomy for sleep apnea and recurrent tonsillitis. He has a significant personal bleeding history. Regular episodes of epistaxis since childhood have required hospital admission, Otorhinolaryngology review , packing and nasal cauterization on 4 occasions. In addition, he has also been troubled by extensive spontaneous bruising and prolonged bleeding from minor wounds that have necessitated clinical review. Although he has not undergone any previous major surgery, 3 previous dental extractions were associated with bleeding. For 2 of these extractions, the prolonged bleeding required packing and resuturing. Based on his bleeding history, this patient has a significantly elevated ISTH BAT score of 11 (positive BS ≥4 for men). Besides his BS, the remainder of his clinical history and physical examination were unremarkable. Despite his bleeding phenotype, all first- and second-line testing detailed in Table 2 was normal, and consequently, he was registered with a diagnosis of BDUC. The planned tonsillectomy will be his first major surgical hemostatic challenge.
Discussion of case 2
BDUC is a diagnosis by exclusion wherein the only positive criterion is that the patient must have a significant bleeding history.1,17 This presents significant challenges when it comes to estimating bleeding risk and monitoring therapy in patients with BDUC undergoing elective procedures. Previous studies have reported significantly enhanced peri-procedural bleeding in subjects with BDUC.6,37 In particular, increased bleeding associated with surgical or dental challenges performed before BDUC diagnosis in the absence of any hemostatic cover has been described.6,37 In a prospective study of 796 patients with VWD, Federici et al38 previously reported that an Molecular and Clinical Markers for the Diagnosis and Management (MCMDM) BAT score >10 was useful in predicting future bleeding risk. Similarly, a significant association between BAT score and subsequent surgical bleeding has also been observed in patients with inherited PFD.39 Because BS is the only identifiable abnormality in patients with BDUC, recent studies have examined whether BATs may be useful in predicting bleeding risk in this context. Relke et al7 conducted a retrospective analysis of 90 adult patients registered with BDUC, all of whom had an abnormal ISTH BAT at diagnosis (mean BS, 10). During follow-up, 58% of this BDUC cohort developed spontaneous bleeding. Interestingly, in keeping with the VWD data, multivariate regression analysis demonstrated that patients with BDUC with higher BS had significantly increased risk for developing a future bleeding event.7 Although these data are interesting, further adequately powered prospective trials will be essential to determine how BS can be used in guiding perioperative hemostatic plans for patients with BDUC. The inherent limitations of BATs are again important when considering their utility for guiding treatment planning for individual patients with BDUC. This is particularly relevant in making peri-procedural plans for younger patients with BDUC, as well as those who may not previously have undergone significant hemostatic challenges.10 As for diagnosis, clinical gestalt and involvement of experienced hemostasis experts are thus again essential in developing perioperative management plans. Based on his bleeding history, our index case has a highly abnormal BAT and is undergoing a procedure associated with high risk of bleeding. He will thus require hemostatic cover for his tonsillectomy.
Treatment options for this patient
There is minimal evidence to guide hemostatic treatment plans for patients with BDUC undergoing minor or major surgical procedures. The limited available data suggest increased peri-procedural bleeding in patients with BDUC who are not treated.6,37 Treatment options that have been used for patients with BDUC include antifibrinolytic agents (tranexamic acid [TA] or aminocaproic acid), desmopressin (DDAVP), platelet transfusion, and recombinant activated FVII (rFVIIa) (Figure3).
TA is widely used in the prevention and treatment of bleeding in BDUC.6,37 Recent large randomized trials have demonstrated that the thromboembolic risk associated with TA use is low, even in high-risk clinical settings such as postpartum or after major orthopedic surgery.40,41 However, it is important to emphasize that the efficacy of TA in maintaining perioperative hemostasis in BDUC remains largely unproven. Nonetheless, our practice is to use antifibrinolytic therapies in all patients with BDUC undergoing minor or major surgical procedures, unless specific contraindications (eg, hematuria or history of thrombosis) are present.
DDAVP is a synthetic analog of vasopressin that causes a transient increase in plasma VWF and FVIII levels.42,43 In addition, DDAVP also has been reported to have additional prohemostatic effects that may be useful in patients with BDUC. Because DDAVP can cause fluid retention, dilutional hyponatremia, and seizures, fluid intake is generally restricted to 1.5 L in the 24 hours after DDAVP, and sodium levels are monitored daily.44 In addition, DDAVP is avoided in children younger than 2 years and in those with a significant history of cardiovascular disease.44
Finally, both platelet transfusion and rFVIIa have been used to prevent or treat bleeding complications in patients with BDUC.30 The efficacy of rFVIIa in this context remains unproven. Consequently, in view of the associated thrombotic risk,45 rFVIIa is generally only considered as a last-ditch measure. For patients with BDUC who have undergone previous minor or major procedures, our practice is to review their clinical records with respect to prior hemostatic treatment plans and their clinical efficacy. Unfortunately, our index case has not undergone any previous significant hemostatic challenges after treatment administration.
Efficacy of treatment in BDUC
Management of patients with BDUC undergoing major surgery continues to pose a significant challenge because there is minimal evidence to guide practice. MacDonald et al6 recently reported retrospective data on 124 patients with BDUC. Of this cohort, 85 (69%) had undergone a surgical procedure before their BDUC diagnosis.6 Importantly, 75% of these procedures were associated with increased postoperative bleeding. Subsequent to BDUC diagnosis, a further 53 minor (including 16 dental extractions) and 16 major procedures were undertaken with hemostatic cover.6 TA alone was used for 22 of the minor procedures, and TA combined with DDAVP was used for a further 24 cases. The other procedures were covered with DDAVP alone (n = 5) or TA in combination with platelets (n = 2). Postprocedure bleeding complications were reported in only 3 (5.7%) cases. Of note, 2 of these patients who developed bleeding had been treated with TA alone before their procedure. In contrast, for the 16 major procedures, no bleeding complications were reported.6 Similarly, Obaji et al37 reported on 78 hemostatic challenges performed in 33 patients with BDUC. Twenty-eight (36%) of these procedures were covered with TA alone, 2 (2%) with DDAVP alone, and 45 (58%) with TA in combination with DDAVP. Minor postprocedural bleeding was observed in 8 (10%) patients with BDUC. In 4 of these cases, the patients had been treated with TA alone before their procedure and bleeding responded to DDAVP infusion.37 Collectively, these limited data suggest that TA in combination with DDAVP is effective for maintaining perioperative hemostasis in most patients with BDUC.
Because our index case has no specific contraindications, we would treat with TA and DDAVP for his tonsillectomy. TA tablets should be started on the evening before the procedure at a dose of 1g 3 times per day. Alternatively, the first dose of TA can be administered intravenously at the time of general anesthetic. Postoperatively, the TA should be continued for 7 to 10 days. Intravenous DDAVP (0.3 μg/kg in 100 mL normal saline) would be infused over 30 minutes immediately before the surgery. DDAVP may also be administered subcutaneously to achieve similar effects (off-label in the United States). Because laboratory tests in this patient are all normal, there is no benefit to repeating hemostatic laboratory investigations after DDAVP. Should the patient develop any significant bleeding complications, treatment options would include repeat DDAVP, platelet transfusion, or rFVIIa. Although DDAVP treatment can be repeated at 12- to 24-hour intervals, tachyphylaxis in terms of attenuated VWF and FVIII responses have been described. In view of the risk of fluid retention, our practice is to confirm that serum sodium levels remain within the normal range before any further DDAVP infusions. If further DDAVP cannot be given, or the bleeding fails to respond, we would treat with platelet infusion.
Management of pregnancy in BDUC
Case 3
A 28-year-old woman with a diagnosis of BDUC is referred at 24 weeks of gestation in her second pregnancy. She has a significant personal bleeding history (including easy bruising and HMB since menarche). At diagnosis, her ISTH BAT score was calculated at 7. Her first pregnancy led to a spontaneous vaginal delivery at 40 weeks of gestation. No hemostatic treatment was given before delivery. Unfortunately, the patient experienced a PPH occurring 12 hours after delivery. The PPH was associated with an estimated blood loss of 1000 mL and required treatment with TA, DDAVP, and packed cell transfusion.
Discussion of case 3
Common causes of PPH include local factors such as uterine atony, lacerations, retained placenta or clots, and systemic bleeding disorders.46 Previous studies suggest that women with BDUC are at increased risk for developing PPH. For example, in a study of 79 women with BDUC who had at least 1 previous delivery, PPH was reported in 50 (63%) patients.6 Similarly, in another retrospective study, Obaji et al37 observed that PPH occurred in 13 of 22 (59%) of women with BDUC. Nine of these patients required transfusion support (with packed cells, fresh frozen plasma, or cryoprecipitate), and 2 women progressed to emergency hysterectomy. Although the numbers are limited, these findings highlight that PPH may be important in women with BDUC. The data are similar to recent studies in women with low VWF, where increased risk of both primary (blood loss ≥ 500mL within 24 hours postpartum) and secondary PPH (excessive bleeding between 24 hours and 12 weeks postpartum) were also seen.47,48
Given the lack of evidence, management of pregnancies in women registered with BDUC continues to pose significant clinical challenges. Treatment options again include TA, DDAVP, platelet transfusion, and rFVIIa. Because fibrinolytic activity is increased during the postpartum period, TA is widely used to prevent PPH in women with mild bleeding disorders.49 Use of prophylactic TA has been shown to reduce the risk of secondary PPH risk in women with VWD.50 Importantly, recent studies have demonstrated that TA is not associated with any significant increase in thrombotic risk during the postpartum period.41 Although some TA is secreted into breast milk, the concentration is considered too low to affect the baby.51 Although previous concerns were expressed regarding the possibility that DDAVP may be associated with a potential oxytocic effect, it has a 1000-fold higher affinity for vasopressin type 2 compared with type 1 receptors. Previous data suggest that DDAVP can also be used safely in women with bleeding disorders during pregnancy and in the postpartum period.52,53 With respect to the specific management of BDUC, MacDonald et al6 recently reported on 13 deliveries in women, including 10 vaginal deliveries and 3 Caesarean sections. These deliveries were managed using a number of different peripartum treatments, including TA alone (n = 6), DDAVP alone (n = 1), TA and DDAVP (1), or TA and platelet transfusion (n = 5). Interestingly, PPH (0.65-1.5 L) was reported in 3 of these cases despite their hemostatic treatment.6
For our index case, whenever possible, we would recommend that her pregnancy should be managed in a center with expertise in the management of bleeding disorders. A written hemostatic management plan should be developed in liaison with the obstetric team and discussed with the patient, her primary care physician, and all members of the multidisciplinary team. In view of her BDUC, we would recommend that neuraxial and spinal anesthesia be avoided if possible. In addition, intramuscular injections and NSAIDs should also be avoided. Her BDUC diagnosis would not influence decisions regarding mode of delivery. In view of her previous primary PPH, we would start on TA 1 g 3 times per day at time of delivery and continue for a further 7 to 10 days postpartum. If the patient develops bleeding complications while on antifibrinolytic therapy, we would treat with DDAVP (0.3 μg/kg in 100 mL normal saline). Platelet transfusion would be considered third-line treatment or if there is a contraindication to DDAVP. For women with BDUC who have previously developed bleeding complications (including PPH) despite being on TA treatment, we would use a combination of TA and DDAVP at time of delivery. Fluid restriction in this peripartum period needs to carefully monitored. Subsequent daily DDAVP infusions may also be required depending on whether adequate postpartum hemostasis has been achieved with normal daily sodium levels. The index patient would be informed that BDUC appears to be heritable in at least some families but that no specific hemostatic testing is indicated for the neonate. Finally, we would discuss the risk of secondary PPH and advise the patient to go to the emergency room if she develops significant bleeding despite TA after her discharge from hospital.
Cases of venous thromboembolism have been described in patients with mild mucocutaneous bleeding disorders including BDUC.6 Consequently, all patients with BDUC undergoing delivery or surgery should routinely be assessed for thrombotic risk. The balance of bleeding vs thrombotic risk in each case needs to be considered on an individual basis by an experienced clinician with hemostasis expertise. Thromboembolic deterrent stockings should be fitted, and early mobilization is encouraged. In addition, standard thromboprophylaxis should be considered once adequate periprocedural hemostasis has been achieved.
Conclusions
It is clear that patients commonly have significant bleeding phenotypes despite the fact that extensive hemostasis laboratory testing is entirely normal. With increasing awareness regarding the prevalence and morbidity associated with HMB and PPH, together with the widespread use of BAT scores to objectively assess bleeding, it seems likely that the number of patients registered with BDUC will continue to rise. For the individual patient, formal BDUC diagnosis is important because it recognizes the significance of the bleeding problem but also has other important implications (Table 3). In addition, the diagnosis should facilitate access to appropriate health care expertise so that careful consideration of risk/benefit assessment can be applied for future potential bleeding risks. With innovations in hemostasis diagnostic testing, further insights into the underlying pathobiological mechanisms in some patients with BDUC will likely continue to emerge over time.54,55 However, it is clear that the hematology community needs to unite to address this neglected area as a matter of urgency. In particular, future international collaborative prospective studies will be required to define optimal diagnostic approaches for BDUC in both children and adult patients. Such studies are will also provide the groundwork on which we can develop an evidence base regarding the safety and efficacy of specific treatment strategies for the clinical management of BDUC.
Authorship
Contribution: R.I.B. and J.S.O. were involved in writing and reviewing the paper.
Conflic-of-interest disclosure: J.S.O. has served on the speaker’s bureau for Baxter, Bayer, Novo Nordisk, Boehringer Ingelheim, Leo Pharma, and Octapharma; has served on the advisory boards of Baxter, Bayer, Octapharma CSL Behring, Daiichi Sankyo, Boehringer Ingelheim, and Pfizer; and has received research grant funding awards from Baxter, Bayer, Pfizer, and Novo Nordisk. R.I.B. has served on the speaker’s bureau for Bayer and on the scientific advisory boards of Roche and Janssen-Cileg. R.I.B.’s institution has received research grant/clinical trial funding from Bayer, Takeda, Pfizer, Daiichi Sankyo, CSL Behring, Roche, Amgen, Celgene, Rigel Pharmaceuticals, Abbvie, Sanofi, MorphoSys AG, Acerta Pharma, Jansen-Cileg, Bristol-Myers Squibb, Boehringer Ingelheim, Portola, Technoclone, and Alexion.
Correspondence: Ross I. Baker, Western Australia Centre for Thrombosis and Haemostasis, Perth Blood Institute, Murdoch University, 90 South St, Murdoch, Perth 6150, Australia; e-mail: ross@pbi.org.au.