Key Points
In males treated with HU during childhood, no toxic effect was found on sperm parameters in addition to baseline impairment.
In boys with severe disease requiring HU treatment before puberty, toxicity for spermatogenesis may not be a major concern.
Abstract
Sperm parameters are known to be impaired in men with sickle cell disease (SCD). Although treatment with hydroxyurea (HU) has an impact on sperm quality, sperm preservation is impossible before puberty. This study’s primary objective was to analyze and compare sperm parameters in male patients with SCD exposed (or not) to HU before puberty. Twenty-six sperm samples from 15 patients (median age, 17 years; range, 16-23) treated with HU during childhood were compared with 46 samples from 23 HU-naïve patients (20 years; 16-24). The median age at HU initiation was 6 years (1-14 years), the median duration of HU treatment was 4 years (0.5-10), and the mean dose of HU was 22.4 ± 3.7 mg/kg per day. Although we observed substantial quantitative and qualitative semen abnormalities in all patients, there were no significant differences in semen volume, sperm concentration, total sperm count, or spermatozoa motility, morphology, and vitality between the HU-exposed and HU-naïve groups. At the time of the semen analysis, 100% of the patients in the HU-exposed group and 52% of the patients in the HU-naïve group received transfusion therapy. The specific effect of HU on spermatogenesis in very young infants and the putative value of transfusion for reversing the toxicity of HU warrant further investigation.
Introduction
In recent years, the very high likelihood of survival to adulthood has emphasized the importance of reproductive issues in patients with sickle cell disease (SCD); however, it has long been established that semen quality is significantly impaired in males with SCD.1 Impairment in at least 1 sperm parameter is found in almost all of these patients, and up to 40% have an abnormal total sperm count.2,3 These findings have been attributed to a variety of causes, including hypogonadism, testicular ischemia, and repeated episodes of fever and hypoxia.4
Along with SCD-related impairments in sperm parameters, sperm quality is further impacted by the cytotoxic effect of hydroxyurea (HU), which is the main disease-modifying drug in SCD. In men, HU causes a significant, rapid, unpredictable impairment in spermatogenesis.3,5 This impairment might be reversed, at least in part, when HU is withdrawn; however, this hypothesis is based on only a few longitudinal studies.3,6 Although sperm abnormalities do not necessarily result in infertility, in Europe sperm banking is recommended before initiating HU in patients with SCD.
According to current guidelines in the United States and the United Kingdom, HU can be offered to children older than 9 months of age, regardless of the clinical severity of SCD. However, uncertainty regarding the toxicity of HU on spermatogenesis in boys is still a concern for parents and health care providers. This study’s primary objectives were to analyze sperm parameters after HU withdrawal in adult males with SCD who were exposed to HU before puberty and to compare the frequencies of abnormalities with those observed in unexposed SCD males.
Study design
We included all male patients with SCD who underwent a semen analysis for sperm banking between February 2007 and October 2019 in 3 major fertility centers in the Paris area of France. To rule out the confounding factor of age on sperm quality, we excluded patients older than age 25 years.7 Next, we retrospectively identified patients who had initiated treatment with HU before puberty (hereafter, the “HU-exposed” group) and patients about to start treatment (the “HU-naïve” group).
We analyzed semen parameters, the main clinical characteristics, the number of vaso-occlusive events (VOEs) in the year before sample analysis, the total number of episodes of acute chest syndrome (ACS), the indication for HU, the duration and dose of HU treatment, and any concomitant medications taken before the semen analysis.
Sperm parameters were assessed according to the 2010 World Health Organization guidelines (ie, semen volume ≥1.5 mL, sperm concentration ≥15 million per milliliter, total sperm count ≥39 million per ejaculate, progressive motility ≥32%, total motility ≥40%, and viability >58%.8 Morphology was rated as the percent of typical forms >23%, as described by Auger et al.9 In most cases, more than 1 sperm sample had been collected for sperm banking, as recommended.
This study was performed in accordance with the French National Data Protection Commission’s MR 004 protocol (reference: 2018DIA007). In line with the French legislation on retrospective studies of routine practice, approval by an institutional review board was not required, and patients did not have to provide their written consent.
Quantitative data were described as the mean ± standard deviation (SD) or the median and range. All statistical analyses were performed by a Mann-Whitney U test or Fisher’s exact test using GraphPad Prism software (version 5.0, GraphPad Software, San Diego, CA). The threshold for statistical significance was set to P < .05. The data for each parameter are expressed as the percentage of patients with an abnormal value.8
Results and discussion
The main characteristics of the study population are summarized in Table 1. All patients had an SS genotype. Fifteen of the participants had been treated with HU before puberty (the HU-exposed group). The median age at initiation of HU was 6 years (range, 1-14 years), the median duration of HU treatment was 4 years (range, 0.5-10 years), and the mean dose of HU was 22.4 ± 3.7 (SD) mg/kg per day. Ten patients (75%) had banked their sperm for fertility preservation before stem cell transplantation. Three patients were determined by their physicians to be intermittently noncompliant with treatment. The HU-naïve group comprised 23 patients; the mean age at the time of the semen analysis was slightly but significantly greater than that of the HU-exposed group. Although the 2 groups did not differ significantly in terms of the frequency of VOEs, ACS was more frequent in the HU-exposed group. At the time of the semen analysis, 100% of the patients in the HU-exposed group and 52% of the patients in the HU-naïve group were receiving transfusion therapy. The indications for the initiation of HU and transfusion therapy are detailed in Table 1.
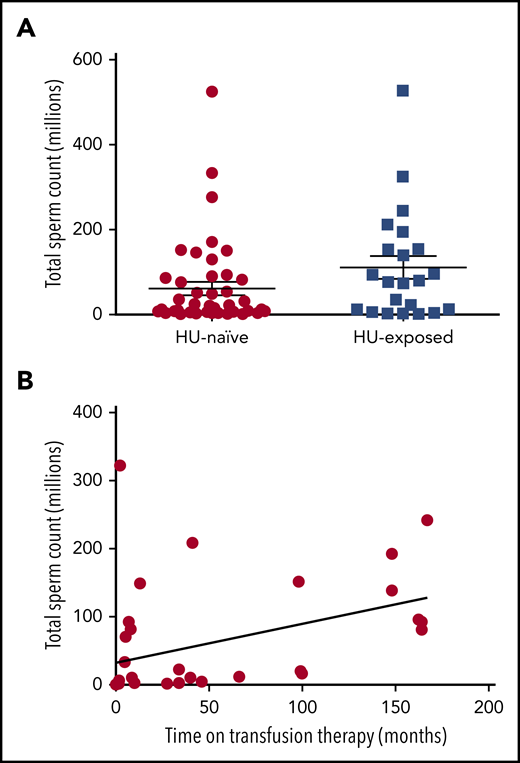
A total of 26 sperm samples in the HU-exposed group and 46 sperm samples in the HU-naïve group were analyzed. Although all patients displayed substantial quantitative and qualitative semen abnormalities, it is noteworthy that there were no significant differences in sperm parameter abnormalities between the 2 study groups (Table 1; Figure 1A). In the HU-naïve group, the sperm abnormalities were in line with those in the literature, confirming that spermatogenesis is impaired in males with steady-state SCD. It is also noteworthy that no cases of azoospermia were observed in either group. The same abnormalities were observed in the HU-exposed group; hence, despite the continuous division of spermatogonia during this quiescent childhood period, HU did not have an irreversible additional cytotoxic effect (ie, in addition to SCD) on spermatogenesis. In fact, oligospermia (defined as <20 million spermatozoa per ejaculate) was less frequent in the HU-exposed group (2 [13%] of 15 patients) than in the HU-naïve group (11 [48%] of 23 patients).
The total sperm count in HU-exposed and HU-naïve male patients with SCD. (A) The relationship between transfusion and semen parameters. (B) The graph shows the positive correlation between the total sperm count of individual semen samples and the duration of transfusion therapy before semen analysis in all transfused patients. (Spearman’s r = 0.4725; P = .0042).
The total sperm count in HU-exposed and HU-naïve male patients with SCD. (A) The relationship between transfusion and semen parameters. (B) The graph shows the positive correlation between the total sperm count of individual semen samples and the duration of transfusion therapy before semen analysis in all transfused patients. (Spearman’s r = 0.4725; P = .0042).
The effect of transfusion on sperm quality is an important issue.10 In HU-exposed patients and in all transfused patients (whether they were exposed to HU or not), we observed a positive correlation between the sperm count and the duration of transfusion (Figure 1B). In the HU-naïve group, the relationship between transfusion and sperm parameters was not significant but the sample size was small. We hypothesize that transfusion therapy may be beneficial (and possibly necessary for the recovery of spermatogenesis) in patients treated with HU during childhood. Larger studies will be needed to address this question.
We did not observe a correlation between sperm parameters and age at HU initiation, possibly because of the small sample size. Likewise, the sperm parameters were not correlated with the duration of HU treatment or the HU dose (for which data were available for only 75% of the patients). Lack of compliance with treatment protocols may have influenced the results, along with the dosing strategy recommended in France (which targets clinical efficacy rather than the maximum tolerated dose). Greater toxicity might be observed at the maximum tolerated dose.
Although this exploratory study was limited by its small sample size, our results show that an irreversible toxic effect on spermatogenesis may not be a major concern in boys with severe disease requiring HU treatment before puberty; the drug’s many beneficial effects largely outweigh its main negative effect in males.11 The effect of HU exposure on infants is still a relevant open question, given that a “mini-puberty” characterized by the multiplication of spermatogonia cells occurs between the ages of 12 and 18 months. So the true risk-benefit ratio of HU initiation during this period in asymptomatic infants is still in question. It also remains to be determined whether the recovery of spermatogenesis in boys treated with HU in childhood necessarily requires transfusion therapy.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Marianne Delville for her help with statistical analysis and Nicolas Garcelon for assistance with the use of Dr Warehouse (the Imagine Institute’s data science platform).
Authorship
Contribution: L.J. was in charge of patients, collected and analyzed the data, and helped write and revise the manuscript; S.M. collected the data, performed statistical analysis, and helped revise the manuscript; C.J., C.C., and I.B. were responsible for the sperm analysis and helped revise the manuscript; C.A., A.K., C.P., A.H., F.B., S.A., M.d.M., B.B.-F., J.-B.A., B.K., M.C., F.L., and J.-A.R. were in charge of patients and helped revise the manuscript; and V.B. designed the study, was in charge of patients, analyzed the data, helped write and revise the manuscript, and was responsible for the study.
Conflict-of-interest disclosure: L.J. is a consultant for Novartis and bluebirdbio. V.B. is a consultant for bluebirdbio, Addmedica, and Astra-Zeneca. The remaining authors declare no competing financial interests.
Correspondence: V. Brousse, Hematology Unit, Sickle Cell Disease Center, Robert Debré Hospital, AP-HP, 19 Boulevard Sérurier, 75019 Paris, France; e-mail: valentine.brousse@gmail.com.