TO THE EDITOR:
HIV-associated primary central nervous system (CNS) lymphoma (HIV-PCNSL), an AIDS-defining malignancy, generally develops in patients with severe CD4+ T-cell lymphopenia. HIV-PCNSL encompasses a variety of CD20+ and usually Epstein Barr virus (EBV)–positive B-cell lymphoproliferative disorders, with a range of polymorphic and monomorphic morphologies.1-3 The World Health Organization classification also recognizes a form of primary diffuse large B-cell lymphoma of the CNS in patients without recognized immunodeficiencies that is usually EBV− and likely has a different natural history.4 Overall survival (OS) of patients with HIV-PCNSL before the advent of antiretroviral therapy (ART) was <2 months, and although OS has improved with ART-associated immune reconstitution, median OS is generally reported to be <1 year.5-8
Before effective ART, the principal mode of treatment of HIV-PCNSL was palliative whole-brain radiotherapy, resulting in long-term neurotoxicity.9,10 Modern ART has made it possible to treat patients with HIV-PCNSL with curative intent; however, radiation-sparing approaches have not been studied prospectively.11 Several cytotoxic chemotherapy approaches have been retrospectively shown to prolong survival in HIV-PCNSL.10,12,13 High-dose methotrexate (HD-MTX) with leucovorin rescue and rituximab is relatively nonmyelotoxic and has been used successfully in other HIV-associated lymphomas.14 Against this background, we initiated a prospective phase 2 study conducted in the HIV and AIDS Malignancy Branch at the National Cancer Institute with HD-MTX, rituximab, and ART for patients with untreated HIV-PCNSL.
Adults with HIV and pathologically confirmed primary CNS B-cell lymphoma were eligible. If tissue diagnosis was not feasible, a positive brain 18F-fluorodeoxyglucose positron emission tomography scan plus EBV+ polymerase chain reaction test of the cerebrospinal fluid (CSF) was acceptable for diagnosis.15 All participants received concurrent ART and appropriate antimicrobial prophylaxis.16 Induction treatment included rituximab (375 mg/m2) IV on day 1 followed by HD-MTX (6 g/m2) IV and leucovorin rescue beginning on day 2 every 2 weeks for 6 cycles. Participants with a complete response (CR) received 2 consolidation cycles of rituximab plus HD-MTX. Patients ineligible to receive HD-MTX at enrollment received ART, rituximab, and best-available chemotherapy. Participants without a CR could receive second-line treatment as part of the protocol. Responses were evaluated using the International Primary CNS Lymphoma Collaborative Group guidelines for response assessment of aggressive non-Hodgkin lymphomas.17,18 Neurocognitive outcomes were measured prospectively with the Folstein Mini Mental Status Exam (MMSE) and formal neurocognitive testing.19,20 The primary objective was to estimate the percentage of participants who had neither recurrent lymphoma nor severe general cognitive dysfunction at 2 years. Response to treatment, immune reconstitution, and OS were evaluated using descriptive statistics and Kaplan-Meier methodology. The protocol was approved by the National Cancer Institute Institutional Review Board (registered at www.clinicaltrials.gov as #NCT00267865). Patients or a durable power of attorney for health care provided written informed consent in accordance with the Declaration of Helsinki.
Twelve participants enrolled between September 2006 and June 2016 (Table 1). Median time from HIV diagnosis to PCNSL diagnosis was 30 weeks (range, 0-23 years), and median CD4+ T-cell count at HIV-PCNSL diagnosis was 16 cells per µL (range, 0-409 cells per µL). Ten participants had baseline CD4+ T-cell counts <100 cells per µL. Four participants were on ART before HIV-PCNSL diagnosis, with 3 of the 4 on ART <4 months. Tissue diagnosis of B-cell lymphoma was confirmed by biopsy in 11 participants and by 18F-fluorodeoxyglucose positron emission tomography scan and elevated EBV viral load in the CSF in 1 participant. All tumors were EBV+, except in participant 2, whose HIV was suppressed on ART for 3 years before diagnosis and who had 409 CD4+ T cells per µL at baseline. Flow cytometry of the CSF revealed leptomeningeal involvement in 4 participants. Three participants had concurrent CNS infections, including Cryptococcus, histoplasmosis, and tertiary syphilis, and 1 had cytomegalovirus retinitis (supplemental Table 1, available on the Blood Web site). One had Pneumocystis jirovecii pneumonia, 2 had secondary syphilis, 1 had disseminated molluscum contagiosum, 1 had esophageal candidiasis, and 3 had cytomegalovirus viremia without end-organ damage. Two participants had active hepatitis B infection with elevated quantitative hepatitis B viral DNA at enrollment. One participant had concurrent Kaposi sarcoma.
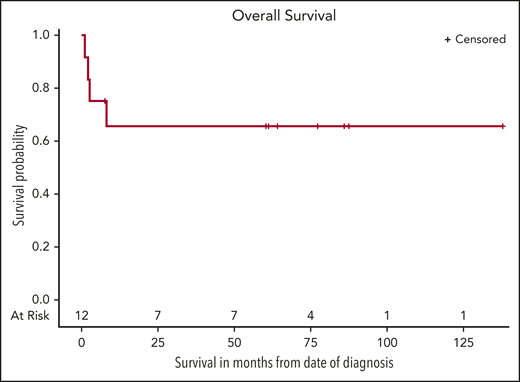
Nine participants completed rituximab plus HD-MTX induction (Table 1). Four had a CR and received 2 cycles of rituximab plus HD-MTX consolidation, with a sustained CR. Also, participant 5, who was initially ineligible to receive HD-MTX because of renal dysfunction, received rituximab with TMZ (150 mg/m2) for 5 days for 1 cycle followed by 6 cycles of rituximab plus HD-MTX plus TMZ and had a CR. Two participants with a PR after induction received second-line rituximab plus TMZ and had a subsequent CR. Participant 2 with an EBV− tumor and high baseline CD4+ T-cell count had a PR after induction and received second-line cytarabine and MTX, with a subsequent CR.21 The last participant with a PR developed a pulmonary embolism and died before receiving further therapy. One participant had progressive disease after induction and subsequently received rituximab plus TMX followed by lenalidomide and whole-brain radiotherapy and died as a result of progressive disease. Two participants received only 1 cycle of therapy and died as a result of rapid disease progression (supplemental Figure 1). Median CD4+ T-cell change from baseline to completion of rituximab plus HD-MTX induction was +35 cells per µL (range, −54 to +369 cells per µL; Table 1; supplemental Figure 2). Overall, 8 (67%) of the 12 participants had a sustained CR, including 3 who received second-line therapy without relapse at 2 years (Figure 1). For all 12 participants, the estimated 60-month OS was 67% (95% CI, 32% to 86%), and with median potential follow-up of 82 months, median OS was not reached. There were no grade 5 adverse events attributable to study therapy (supplemental Table 2). The most common adverse events were hematologic, including grade 4 neutropenia, experienced by 83% of participants. Grade 4 leukopenia (42%) and CD4+ lymphopenia (33%) were also common, although CD4+ T cells increased overall throughout induction treatment. No participants developed opportunistic infections during therapy.
Ten participants underwent baseline MMSE evaluation, with median score of 22 (mild dementia) and a range of 5 (severe cognitive impairment) to 29 (normal; Table 1). The MMSE was repeated after induction treatment in 7 participants; early neurologic improvement was noted, with median score of 28 (range, 27-30) and median increase of +7 points from baseline. Five participants underwent full neurocognitive testing after completion of induction treatment (supplemental Table 3). All 5 participants included in the analyses improved in at least 1 cognitive domain, and 4 of the 5 improved in 1 or both processing speed tasks. Only 2 participants showed decline across any of the 5 domains, and each of these patients declined in only 1 domain. All 8 participants with a sustained CR were free of severe general neurocognitive dysfunction at 24 months and able to live on their own unassisted.
Our study is the first to prospectively evaluate the responses and long-term neurocognitive effects of radiation-sparing therapy in HIV-PCNSL. All surviving participants were free of severe general neurocognitive dysfunction after treatment, and the surviving participants who were tested had improvement in most domains of cognition in the 3 years after treatment. Our study provides the first prospective evidence that treatment with ART and rituximab plus HD-MTX is associated with a high response rate, CD4+ lymphocyte reconstitution, and long-term survival with preservation or improvement of neurocognitive function. The treatment regimen is tolerable, even in high-risk patients with advanced HIV, significant comorbidities, and CNS infections. Curative treatment with ART and rituximab plus HD-MTX is an appropriate standard first-line therapy for HIV-PCNSL.
Presented in part in abstract form at the 15th International Conference on Malignancies in HIV/AIDS, Bethesda, MD, 26-27 October 2015; the American Society of Hematology Meeting on Lymphoma Biology, Washington, DC, 2-5 August 2018; and the 60th annual meeting of the American Society of Hematology, San Diego, CA, 1-4 December 2018.
Primary data for this study are available in clinicaltrials.gov under identifier NCT0026786. For other original data not found in clinicaltrials.gov, please contact Kathryn Lurain at kathryn.lurain@nih.gov.
The online version of this article contains a data supplement.
Acknowledgments
The authors thank Kirsta Waldon for research and clinical care coordination; Deirdre Mahoney; the nurses, physicians, and support staff of the National Cancer Institute Medical Oncology Service; and our patients and their families.
This research was supported by the Intramural Research Program of the National Cancer Institute (NCI), National Institutes of Health (NIH), and by US federal funds from the NCI, NIH, under contract HHSN261200800001E.
Authorship
Contribution: R.F.L. and R.Y. designed the study; K.L., T.S.U., M.N.P., A.W., R.R., P.H.G., R.F.L., J.G., and R.Y. cared for patients; E.S.J., S.P., C.M.Y., and H.-W.W. provided pathologic conformation of cases; K.L., T.S.U., and P.H.G. collected data; P.L.W. and S.M. planned the neurocognitive test battery and interpreted the results; M.A.T. and S.M. performed the neurocognitive testing; S.M.S., K.L., T.S.U., R.R., and R.Y. analyzed data; and all authors contributed to writing and approving the manuscript.
Conflict-of-interest disclosure: K.L., T.S.U., R.R., and R.Y. report receiving research support from Celgene through a Cooperative Research and Development Agreement (CRADA) at the National Cancer Institute (NCI). R.R., K.L., and R.R. receive research support from EMD-Serono for an ongoing study through a CRADA at the NCI. R.R., T.S.U., K.L., and R.Y. report receiving drug for a clinical trial from Merck through a CRADA with the NCI. T.S.U. reports receiving other commercial research support from Roche through a Clinical Trial Agreement with Fred Hutchinson Cancer Research Center. T.S.U. and R.Y. are 2 of 3 coinventors on US patent 10 001 483 entitled “Methods for the treatment of Kaposi’s sarcoma or KSHV-induced lymphoma using immunomodulatory compounds, and uses of biomarkers.” R.Y. is also a coinventor on patents for a peptide vaccine for HIV and for the treatment of Kaposi sarcoma with interleukin-12 (IL-12), and an immediate family member of R.Y. is a coinventor on patents related to internalization of target receptors, Kaposi’s sarcoma herpesvirus viral IL-6, and use of calreticulin and calreticulin fragments to inhibit angiogenesis. All rights, titles, and interests regarding these patents have been or should by law be assigned to the US Department of Health and Human Services; the government conveys a portion of the royalties it receives to its employee inventors under the Federal Technology Transfer Act of 1986 (P.L. 99-502). The remaining authors declare no competing financial interests.
Correspondence: Kathryn Lurain, National Cancer Institute, 10 Center Dr, Room 6N110, Bethesda, MD 20892; e-mail: kathryn.lurain@nih.gov.