Key Points
In a case-control study, the frequency of thrombosis was higher in patients with MPN with second cancer than in matched MPN controls.
The occurrence of arterial thrombosis was associated with a twofold increased risk of carcinoma.
Abstract
Patients with Philadelphia-negative myeloproliferative neoplasm (MPN) are prone to the development of second cancers, but the factors associated with these events have been poorly explored. In an international nested case-control study, we recruited 647 patients with carcinoma, nonmelanoma skin cancer, hematological second cancer, and melanoma diagnosed concurrently or after MPN diagnosis. Up to 3 control patients without a history of cancer and matched with each case for center, sex, age at MPN diagnosis, date of diagnosis, and MPN disease duration were included (n = 1234). Cases were comparable to controls for MPN type, driver mutations and cardiovascular risk factors. The frequency of thrombosis preceding MPN was similar for cases and controls (P = .462). Thrombotic events after MPN and before second cancer were higher in cases than in controls (11.6% vs 8.1%; P = .013), because of a higher proportion of arterial thromboses (6.2% vs 3.7%; P = .015). After adjustment for confounders, the occurrence of arterial thrombosis remained independently associated with the risk of carcinoma (odds ratio, 1.97; 95% confidence interval, 1.14-3.41), suggesting that MPN patients experiencing arterial events after MPN diagnosis deserve careful clinical surveillance for early detection of carcinoma. This study was registered at www.clinicaltrials.gov as NCT03745378.
Introduction
The clinical burden of the Philadelphia-negative myeloproliferative neoplasm (MPN) is marked by arterial and venous thrombosis, hemorrhagic complications, and a propensity for transforming into myelofibrosis and acute myeloid leukemia.1 In addition, recent cohort studies2-6 and population-based results7-9 highlighted that MPN patients are prone to development of second cancers and lymphoproliferative disorders.7,8
It is well known that unprovoked venous thromboembolism (VTE) may precede a subsequent malignancy,10 but the notion that malignancy can be heralded by arterial thrombosis has been reported only recently.11 To date, this association has not been studied in MPN, in which arterial thrombosis is more frequent than venous thrombosis and solid tumors are reported with a higher frequency.
We recently published the results from a nested case-control study with 647 MPN cases with second cancer and 1234 matched MPN cancer-free patients recruited from European LeukemiaNet centers, reporting the impact of the exposure to cytoreductive drugs on the occurrence of second cancer.12 In the present study, we reexamined this large database with the following 2 purposes: (1) to evaluate the frequency and type of vascular complications in MPN patients with carcinoma, nonmelanoma skin cancer, melanoma, and hematological cancer excluding leukemia, and (2) to establish whether arterial and venous thrombosis registered during follow-up after diagnosis of MPN predicts the occurrence of a second cancer.
Study design and statistical methods
Details of the multicenter international nested case-control MPN-K Study have been reported elsewhere.12
This project was approved by the institutional review board of each participating center.
Cases were MPN patients with a second cancer diagnosed concurrently or subsequent to MPN diagnosis. The date of the second cancer diagnosis was defined as the index date. Controls were MPN patients without a second cancer. For each case, up to 3 cancer-free controls were matched for center, sex, age at MPN diagnosis, date of MPN diagnosis, and MPN disease duration.
The major thrombotic events of interest were ischemic stroke, transient ischemic attack, acute myocardial infarction, unstable angina pectoris, peripheral arterial thrombosis, retinal artery or vein occlusion, deep venous thrombosis (including thrombosis of cerebral and splanchnic veins), and pulmonary embolism. All the events were objectively proven, as previously described.13-15 Thrombosis had to have occurred before or concurrent with MPN diagnosis or in the period after MPN diagnosis and before the index date.12
The χ2 test or Fisher’s exact test (for categorical data) and Student t test or Mann-Whitney U test (for continuous variables) were used when appropriate. The cumulative incidence of thrombosis from MPN diagnosis was estimated by the Kaplan-Meier method and was compared between cases and controls by the log-rank test. A multivariable conditional logistic regression model was fitted to estimate the odds ratio (OR) with 95% confidence interval (CI) of second cancer associated with the occurrence of thrombosis before and at MPN diagnosis and during follow-up. The estimates were adjusted for the effect of age at MPN diagnosis, cardiovascular risk factors (smoke, hypertension, dyslipidemia, and diabetes), JAK2V617F mutation, and treatment during follow-up (primary antithrombotic prophylaxis with aspirin and/or cytoreduction). For all tested hypotheses, 2‐tailed results reaching P < .05 were considered to be significant. Analyses were performed using STATA software, release 13 (StataCorp LP, College Station, TX).
Results and discussion
The most frequent category of cancer among the 647 cases was carcinoma (65.8%; supplemental Table 1, available on the Blood Web site). Carcinoma involved mostly the prostate (n = 121), breast (n = 88), lung (n = 56), or colorectal region (n = 56); the complete details of cancer diagnoses have been reported elsewhere.12 Cases were comparable with the 1234 matched controls for demographics, type of MPN, and potential confounders, such as driver mutations, abnormal karyotype, and cardiovascular risk factors (supplemental Table 2).
Approximately 20% of either MPN cases or controls exhibited thrombosis before MPN or at diagnosis (19.8% vs 21.1%, respectively; P = .462; supplemental Table 3). In contrast, significant differences in the proportion of thrombosis were found after MPN diagnosis. After a median observation time from the diagnosis of MPN to the index date of 4.5 years (interquartile range, 1.5-8.2) in cases and 3.7 years (interquartile range, 1.5-7.5) in controls, a higher percentage of thrombosis was found in cases with respect to controls (75 of 647 [11.6%] vs 100 of 1234 [8.1%], respectively; P = .013). Approximately one-third of the thromboses preceding cancer occurred in the 12 months before the diagnosis of the second cancers (22/75; 29.3%). The excess of thrombosis in cases was due to a higher frequency of arterial thrombosis (40 of 647 [6.2%] vs 46 of 1234 [3.7%]; P = .015), whereas no significant difference was found for venous thrombosis (35 of 647 [5.4%] vs 53 of 1234 [4.3%]; supplemental Table 3). The distribution of thrombosis in the different MPNs is shown in supplemental Table 4.
Among patients with thrombosis, no difference was found between cases and controls in the rate of arterial or venous thrombosis occurring during treatment with hydroxyurea. On the other hand, in cases with second cancer, the proportion of arterial thrombosis in the absence of hydroxyurea, was higher than in the controls (1.5% vs 0.4%; P = .008; supplemental Table 5).
Among the thrombotic events, 125 (71.4%) were new events, and 50 (28.6%) were recurrences of a prior thrombosis. Compared with patients who had no thrombosis during follow-up, patients with recurrent thrombosis had a higher risk of second cancer (OR, 2.13; 95% CI, 1.19-3.81; P = .011), whereas patients with new thrombotic events during follow-up had a similar risk of development of a second cancer (OR, 1.28; 95% CI, 0.86-1.90).
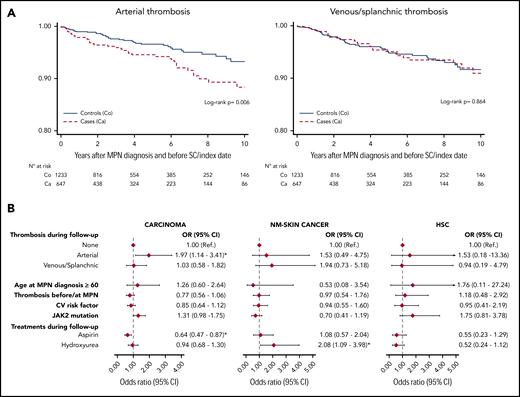
Although the cumulative incidence of venous thrombosis over time was similar among cases and controls (P = .864), the cumulative incidence of arterial thrombosis was higher in cases with a second cancer (P = .006; Figure 1A).
Kaplan-Meier curves and multivariable analysis. (A) Ten-year arterial and venous/splanchnic thrombosis-free curves from MPN diagnosis in cases (Ca) and controls (Co). (B) Effect of arterial and venous/splanchnic thrombosis on risk of second cancer (SC) obtained by a multivariable conditional logistic regression model, adjusted for potential confounders (age, cardiovascular risk factors, JAK2 mutation, and time of thrombosis at diagnosis or during follow-up) and stratified by type of second cancer. Because of the low number of cases, no multivariable model was fitted for melanoma. HSC, hematological second cancer; NM, nonmelanoma.
Kaplan-Meier curves and multivariable analysis. (A) Ten-year arterial and venous/splanchnic thrombosis-free curves from MPN diagnosis in cases (Ca) and controls (Co). (B) Effect of arterial and venous/splanchnic thrombosis on risk of second cancer (SC) obtained by a multivariable conditional logistic regression model, adjusted for potential confounders (age, cardiovascular risk factors, JAK2 mutation, and time of thrombosis at diagnosis or during follow-up) and stratified by type of second cancer. Because of the low number of cases, no multivariable model was fitted for melanoma. HSC, hematological second cancer; NM, nonmelanoma.
The excess of arterial thrombosis after MPN diagnosis was limited to cases with carcinoma (Table 1). Moreover, cases with carcinoma experienced splanchnic vein thrombosis after MPN diagnosis more frequently than controls (Table 1). In the multivariable model, arterial thrombosis during the follow-up was confirmed to be an independent predictor of carcinoma (OR, 1.97; 95% CI, 1.14-3.41; P = .015; Figure 1B). Cardiovascular risk factors or JAK2 mutational status had no impact on the risk of second carcinoma. Low-dose aspirin, used as a prophylaxis for incident thrombosis, showed a significant protective role against the occurrence of carcinoma, as discussed elsewhere.16 Patients on cytoreduction during the follow-up received hydroxyurea in 91.1% of cases (952 of 1045) and had a similar risk of carcinoma and hematological second cancer and a double the risk of nonmelanoma skin cancer vs the untreated patients (Figure 1B).
These findings reveal in MPN patients an association of arterial thrombosis with second cancer. Interestingly, in 2 recent large European LeukemiaNet surveys performed in 387 MPN patients with VTE13,14 and in another study with 597 MPN patients with cerebrovascular ischemic events,15 the frequency of second cancer was 1.7-fold higher in the latter group than in MPN patients with VTE (8.5% vs 4.9%, respectively, P = .036).
The incidence of cancer after VTE in MPN patients was similar to that observed in the general population, where 5.2% of patients with unprovoked VTE develop cancer within 12 months from VTE.11 In contrast, the frequency of second cancer after arterial thrombosis appears greater than in the general population. In a series of 374 331 patients older than 67 years, 1.75% of cancer patients had an arterial thrombotic event within 1 year preceding the diagnosis of cancer, with an increased risk of 69% vs the matched cancer-free controls.12 In 2 other population-based studies, patients with lower limb arterial thrombosis or myocardial infarction had a cancer incidence 1.4-17 and 1.6-fold18 higher than subjects without thrombosis 1 to 3 years after the event. Arterial thrombosis has been reported to be preferentially associated with lung and kidney cancer19 ; however, in our MPN series, lung and kidney cancer accounted for only 13.1% and 1.1% of cases with carcinoma, respectively (supplemental Table 1).
The procoagulant mechanisms underlying the cancer-associated thrombophilia are complex and multifactorial and have been specifically explored to explain the association with venous thrombosis.20 Interestingly, there is evidence that patients with solid tumors have a higher prevalence of clonal hematopoiesis of undetermined significance,21,22 and it is not surprising that MPN clonal diseases may further increase the carcinogenetic risk. A possible biological plausibility for the link between arterial thrombosis and carcinoma in MPN patients may be related to an underlying common pathogenic mechanism, such as an aberrant inflammatory response consistently found in MPN.23
Our observations may have practical implications and suggest careful clinical surveillance for diagnosis of early cancer in MPN patients with arterial thrombosis during the follow-up. The International Society on Thrombosis and Haemostasis guidance for patients with unprovoked VTE could be adopted. This approach includes a careful medical history, physical examination, basic laboratory investigations, and chest radiograph, as well as age- and sex-specific cancer screening (ie, breast, cervical, colon, and prostate).24
For original data, please contact the corresponding author.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This study was supported by Fondazione per la Ricerca Ospedale Maggiore (FROM), Papa Giovanni XXIII Hospital, Bergamo, Italy; and by Associazione Italiana per la Ricerca sul Cancro, Grant 5perMille, Progetto MYNERVA (P.G. and A.M.V.).
Authorship
Contribution: T.B. and V.D.S. conceived and designed the study, collected the data, analyzed and interpreted the data, and wrote the manuscript; A.G. collected the data, performed the statistical analysis, interpreted the data, and wrote the manuscript; all remaining authors collected the data, interpreted the data, and revised the manuscript for important intellectual content; and all authors approved the final version of the manuscript.
Conflict-of-interest disclosure: T.B. has been a speaker and consultant for Novartis and received a research grant from AOP Orphan. V.D.S. has received consulting and lecture fees from Amgen, Celgene, and Novartis and institutional research grants from Bayer and Novartis. M.L.F. has been a member of the advisory board for Novartis, and she has received travel grants from the company. M.F.M. has been a speaker and consultant for Novartis. M.M. has received honoraria for serving on advisory boards and delivering lectures at sponsored meetings from Celgene, Amgen, Janssen, Gilead, and Novartis. A.M.V. has been a speaker for Novartis, Celgene, and Shire, and has served on advisory boards of Celgene, Incyte, and Novartis. The remaining authors declare no competing financial interests.
Correspondence: Tiziano Barbui, FROM Research Foundation, Papa Giovanni XXIII Hospital, Piazza OMS 1, 24127 Bergamo, Italy; e-mail: tbarbui@fondazionefrom.it.