Acute Myeloid Leukemia (AML) is a clonal disease of the hematopoietic system that initiated and sustained by self-renewing hematopoietic stem and progenitor cells (HSPC). Mutations in the de novo DNA methyltransferase 3A (DNMT3A) gene occur in approximately 25% of adult acute myeloid leukemias (AML). Although the mechanisms through which such mutations promote leukemogenesis remain unclear, we have previously shown that loss of the DNMT3A can inhibit normal hematopoietic differentiation (Challen, Nature Genetics, 2011), accounting for the emergence of DNMT3A-HSC clones as a predisposition to hematological malignancies (Yang, Cancer Cell, 2015). Therapies that selectively eliminate the initiating pre-leukemic population would greatly improve outcomes for affected patients. However, the identification as well as selective elimination of such a distinct population has been problematic because of the considerable overlap in gene expression profiles with bulk normal hematopoietic stem cells. Molecular targets during leukemia development have not been well elucidated due to lack of the real definitive markers, which is a significant knowledge gap and barrier for understanding clonal leukemogenesis and therapeutic applications.
Single-cell RNA sequencing has emerged as a powerful tool to analyze new cell types, cellular heterogeneity and cell differentiation routes. This technique made important contributions to our understanding of hematopoietic stem and cancer cell heterogeneity and selective resistance of cancer cell subpopulations to molecularly targeted cancer therapies.
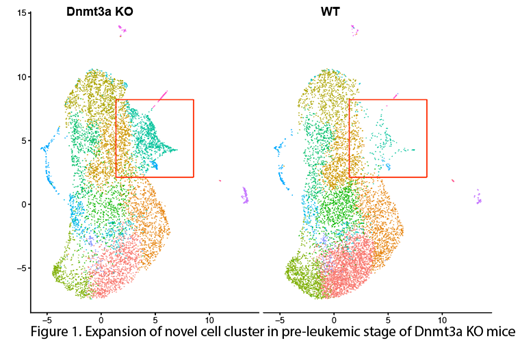
To identify early events involved in pre-leukemic transformation, we have performed single-cell RNA-sequencing (scRNA-seq) in WT and Dnmt3a KO mice. Flow cytometry sorted wild-type and pre-leukemic Dnmt3a KO HSPC cells were captured using 10X genomics chromium platform. After genome mapping, dimensional reduction, and clustering using Cell ranger pipeline, we generated transcriptome data and integrated the data sets using Seurat. Approximately 8,000 cells from each group were sequenced, and each cell expressed 1800-4500 genes. Graph-based clustering analysis revealed 16 unique cell clusters in both WT and DNMT3A KO mice. Interestingly, when compared with WT mice, we observed a 10-fold expansion of a single cell cluster in Dnmt3a KO cells before the advent of overt leukemia. This cluster co-expresses several stem cell genes including well-known leukemic stem cell surface markers such as CD47, as well as several novel genes. Some of these novel genes, encode cell surface proteins such as Ly6c2 and Ly86. We further validated protein expressions in AML cell lines and primary AML blast.
In conclusion, the discovery of novel cluster in DNMT3A KO mice, and the relative abundance of this cluster in pre-leukemic stage of DNMT3A KO mice indicates that they promote leukemogenesis and offers an opportunity to specifically target DNMT3A mutant pre-leukemic cells using T cell immunotherapy.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.