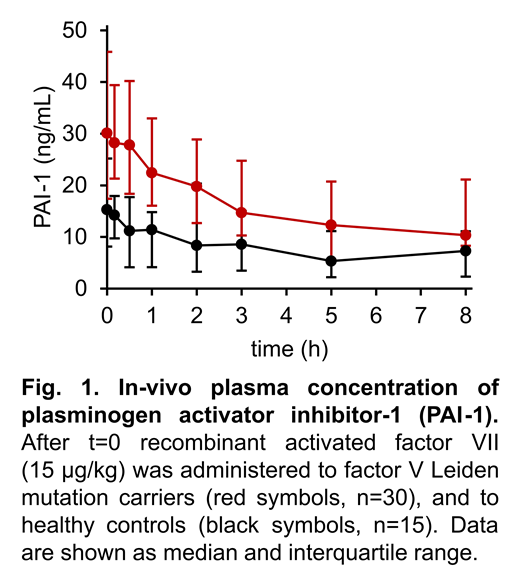
Background: APC has been suggested to contribute to a hyperfibrinolytic state in various acquired coagulation disorders, including coagulopathies induced by trauma and sepsis. However, in vivo evidence for the proposed underlying mechanism of proteolytic degradation of PAI-1 by APC remains inconclusive. Recently, we have shown increased APC generation in response to in vivo thrombin formation in carriers of the factor V Leiden mutation (FVL). In this approach of stimulated hemostasis activity pattern evaluation (SHAPE), in vivo thrombin formation was triggered by low-dose administration of recombinant activated factor VII (rFVIIa). Aim of the present study was to investigate the resulting effects on the fibrinolytic system. Methods: The study population consisted of 30 FVL carriers (thereof 13 with a history of venous thromboembolism) and 15 healthy controls. Blood samples were collected immediately before and during a period of 8 hours following injection of 15 µg/kg rFVIIa. Plasma levels of APC were quantified using an oligonucleotide-based enzyme capture assay (OECA). Other monitored parameters included plasma levels of prothrombin activation fragment 1+2 (F1+2), tissue-type plasminogen (t-PA), PAI-1, plasminogen, α2-antiplasmin, plasmin-α2-antiplasmin complexes (PAP), soluble fibrin monomers, d-dimer, and thrombin-activatable fibrinolysis inhibitor (TAFI). Results: At baseline, FVL carriers showed higher median levels of APC in comparison to the controls (1.39 vs. 0.86 pmol/L, P=.001), higher PAI-1 levels (30.1 vs. 15.3 ng/mL, P=.002) and lower plasminogen levels (94.8 vs. 110.4%, P=6·10-4). The other baseline parameters did not differ significantly. In both cohorts a comparable increase of F1+2 was observed after administration of rFVIIa, whereas APC increased more (P=.004) in FVL carriers (by 6.40 pmol/L) than in healthy controls (by 2.14 pmol/L), Concurrently, median PAI-1 levels decreased more (P=.007) in FVL carriers (by 19.8 pmol/L) than in healthy controls (by 8.0 pmol/L) (Figure 1). TAFI levels decreased temporarily, from 104.8 to 94.4% (P=.007) in FVL carriers and from 105.0 to 92.1% (P=2·10-4) in healthy controls. PAP levels increased from 164 to 209 ng/mL (P<10-4) in FVL carriers and from 154 to 189 ng/mL (P=.023) in the control group. The extent of these changes did not differ between the two cohorts. D-dimer level increased only in FVL carriers, from 0.34 to 0.41 mg/L (P=.008). t-PA and the other parameters did not show significant changes after rFVIIa administration. Conclusion: Increased APC formation rates in FVL carriers were associated with a greater decline of PAI-1 levels in the absence of interfering changes in t-PA levels. These data provide further in vivo evidence that APC down-regulates PAI-1. Overall, the SHAPE approach utilized here does not induce a significant profibrinolytic response, even in patients with thrombophilia.
Reda:Grifols: Honoraria; Shire: Honoraria. Winterhagen:SOBI: Honoraria. Berens:Pfizer: Honoraria; Shire: Honoraria; Biotest: Honoraria; CSL Behring: Honoraria; Novo Nordisk: Honoraria; Baxter: Honoraria. Müller:NovoNordisk: Membership on an entity's Board of Directors or advisory committees; Bayer: Membership on an entity's Board of Directors or advisory committees; Siemens: Membership on an entity's Board of Directors or advisory committees; Roche: Membership on an entity's Board of Directors or advisory committees. Oldenburg:CSL Behring: Consultancy, Research Funding, Speakers Bureau; NovoNordisk: Consultancy, Honoraria, Research Funding; Bayer: Consultancy, Research Funding, Speakers Bureau; Swedish Orphan Biovitrum: Consultancy, Speakers Bureau; Grifols: Consultancy, Speakers Bureau; Takeda (Shire): Consultancy, Research Funding, Speakers Bureau; Chugai: Consultancy, Speakers Bureau; Roche: Consultancy, Speakers Bureau; Biotest: Consultancy, Research Funding, Speakers Bureau; Octapharma: Consultancy, Research Funding, Speakers Bureau; Pfizer: Consultancy, Speakers Bureau. Rühl:CSL Behring: Honoraria; Bayer: Honoraria; Shire: Honoraria; SOBI: Honoraria; Sanofi Genzyme: Honoraria; Grifols: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.