Background and aims. B-lineage acute lymphoblastic leukemia (B-ALL) carrying t(4;11)(q21;q23)/KMT2A-AFF1 accounts for roughly 10% of adult B-ALL, it is associated to pro-B immunophenotype and aggressive clinical features. However, the role of KMT2A-AFF1 in pediatric-like, minimal residual disease (MRD)-driven clinical trials and the impact of transplant in this poor prognostic subgroup are still debated. To address these issues we investigated the incidence, clinical features and outcome of KMT2A-AFF1-positive ALL treated in 6 consecutive GIMEMA (Gruppo Italiano Malattie EMatologiche dell'Adulto) clinical trials.
Materials and methods. Between November 1996 and September 2016, 926 BCR-ABL1-negative B-ALL patients were enrolled in the GIMEMA clinical trials LAL0496, LAL2000, LAL0904, LAL1104, LAL1308, LAL1913. The median follow-up is 25.4 months (range: 0.1-146.9). Starting from LAL2000, KMT2A-AFF1-positive patients were classified as high-risk and managed more intensively. LAL1308 and LAL1913 adopted a similar pediatric-like backbone, at variance from LAL0496, LAL2000 and LAL0904. Overall, median age was 34.3 (range, 14.1 - 81.9), there were 502 males (54.2%) and 424 females (45.8%) and the median WBC count was 8.80 x 109/L (range, 0.4- 872.3). Incidence, clinical features and outcome of KMT2A-AFF1-positive were analyzed in comparison with KMT2A-AFF1-negative B-ALL. MRD evaluation after induction treatment was available in 197 patients enrolled in the more recent protocols.
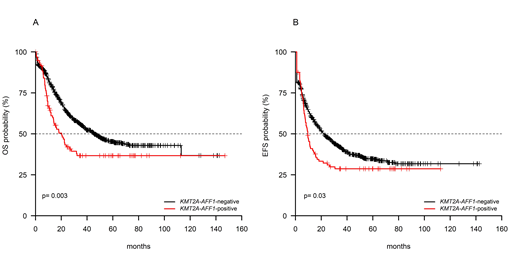
Results. Overall, 97/926 (10.5%) samples harbored KMT2A-AFF1 fusion gene. The comparison between KMT2A-AFF1-positive and the 829 KMT2A-AFF1-negative B-ALL patients revealed that KMT2A-AFF1-positive patients had a higher median age (42 vs 32.5 years old, p<0.001), were more likely to be female (60/97 vs 364/829, p=0.001) and had a significantly higher median WBC count (70.7 vs 7.2 x 109/L, p<0.001). The complete remission (CR) rate did not differ between KMT2A-AFF1-positive and -negative patients (87.5% vs 81.5%, p=0.188). Notwithstanding, at 24 months, KMT2A-AFF1-positive patients had a significantly shorter survival than KMT2A-AFF1-negative in terms of median overall (OS, 20.3% vs 45.5%, p=0.003, Figure 1A), disease-free (DFS, 9.2% vs 34.3%, p<0.0001) and event-free survival (EFS, 9.5% vs 21.9%, p=0.03, Figure 1B). Of the KMT2A-AFF1-positive patients, 25 underwent transplant (18 received an allograft, 7 an autograft) and - in keeping with this - survival analyses were repeated after censoring for transplant and we found that KMT2A-AFF1-positive patients maintained a significantly worse OS (p<0.0001), DFS (p<0.0001), EFS (p=0.036). Similarly, the effect of KMT2A-AFF1-positivity was adjusted for treatment received and retained statistical significance (HR: 1.3, 95%CI: 1.0-1.7, p=0.050). Moreover, when survival analyses were restricted to the only KMT2A-AFF1-positive patients, we did not observe significant differences among the median survival across the various clinical trials (p=0.581). MRD quantification, with the caveat that the number of patients evaluated for MRD is small, showed that KMT2A-AFF1-positive and negative patients did not differ in the achievement of MRD-negativity: indeed, 8/26 (30.8%) KMT2A-AFF1-positive and 71/171 (41.5%) KMT2A-AFF1-negative patients were MRD-negative post-induction.
Conclusions. The present study examined a cohort of 926 BCR-ABL1-negative B-ALL treated in Italian multicenter clinical trials. Overall, though not differing in the achievement of CR and MRD negativity, the analysis of survival curves confirms that KMT2A-AFF1-positive patients have a significantly shorter survival than KMT2A-AFF1-negative patients. This result holds true also when censoring for transplant, thus suggesting that allograft does not confer a survival advantage to KMT2A-AFF1-positive patients. Moreover, KMT2A-AFF1-positive patients perform poorly also in more intensive regimen, as emerged from the analysis of the GIMEMA LAL1913 clinical trial. These findings encourage the adoption of alternative strategies: among the available targeted therapies, preclinical models of KMT2A-leukemia show sensitivity to BCL2-inhibitors, thus providing the rationale for testing this approach in upcoming clinical trials for KMT2A-AFF1-positive patients.
Chiaretti:Amgen: Membership on an entity's Board of Directors or advisory committees; Incyte: Membership on an entity's Board of Directors or advisory committees; Shire: Membership on an entity's Board of Directors or advisory committees; Pfizer: Membership on an entity's Board of Directors or advisory committees. Krampera:Novartis: Membership on an entity's Board of Directors or advisory committees; Janssen: Membership on an entity's Board of Directors or advisory committees. Di Raimondo:Amgen: Consultancy, Honoraria, Research Funding; Takeda: Consultancy. Bassan:Pfizer: Honoraria, Membership on an entity's Board of Directors or advisory committees; Shire: Honoraria, Membership on an entity's Board of Directors or advisory committees; Amgen: Honoraria, Membership on an entity's Board of Directors or advisory committees; Servier: Honoraria, Membership on an entity's Board of Directors or advisory committees; Novartis: Membership on an entity's Board of Directors or advisory committees. Vignetti:Pfizer: Membership on an entity's Board of Directors or advisory committees, Other: Educational Training; Incyte: Membership on an entity's Board of Directors or advisory committees, Other: Educational Training.
Author notes
Asterisk with author names denotes non-ASH members.