Abstract
Chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL) and follicular lymphoma (FL) represent indolent malignancies characterized by multiple episodes of relapse. Therapy has centered on agents that largely target the B-cell receptor pathway. Duvelisib is a second-generation oral inhibitor of phosphoinositide-3 kinase, downstream of the B-cell receptor pathway, approved in the United States for relapsed CLL/SLL and FL. Duvelisib represents a highly active agent, and ongoing investigations, including fixed-duration drug combinations and alternative dosing schedules, are aimed at reducing immune-mediated toxicities.
Introduction to PI3K inhibition and duvelisib
Chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL) and follicular lymphoma (FL) are common indolent hematologic malignancies. Major advances in therapeutics over the last 20 years have improved survival for patients with CLL/SLL and FL; however, these diseases remain largely incurable. Both CLL/SLL and FL are heterogeneous diseases in which outcomes have been shown to be dependent on the disease, molecular, and genetic characteristics, as well as patient-specific features. Recently, several important targeted therapies for CLL/SLL and FL have emerged, including agents that inhibit B-cell receptor (BCR)-associated kinases. Several of them, including Bruton tyrosine kinase (BTK) and phosphatidylinositol 3-kinase (PI3K), play key roles in tonic BCR signaling, and thus, their inhibition antagonizes B-cell survival and proliferation. In particular, the BTK inhibitors have had tremendous success both in treatment-naive and relapsed/refractory (R/R) patients with CLL/SLL. Despite the promise of BTK inhibition in CLL/SLL, many patients will need additional drugs during their lifetime. Moreover, the activity of BTK inhibitors is limited in FL, and none have been approved in FL.
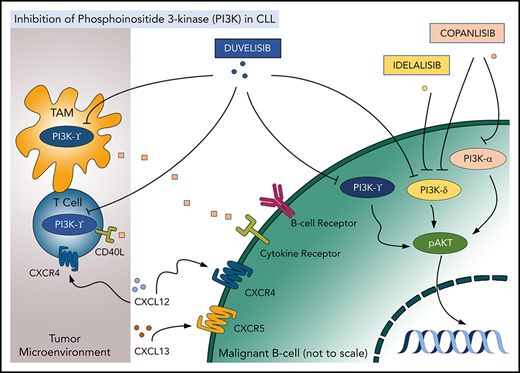
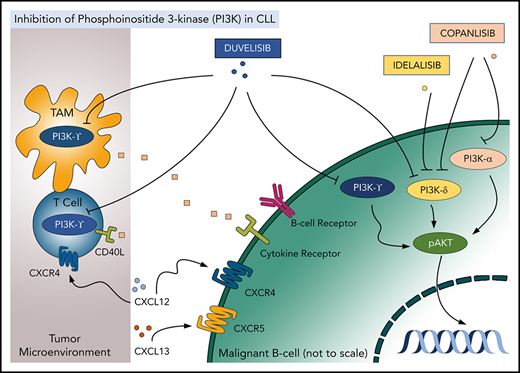
PI3K inhibitors represent an important class of novel CLL/SLL and FL therapies. PI3K is a well-recognized biologic target in cancer. Four class I PI3K catalytic isoforms are expressed in mammalian tissue (p110α, β, γ, and δ).1 While p110α/β isoforms are expressed ubiquitously, p110γ/δ isoforms are predominantly expressed in immune cells. Multiple surface receptor pathways activate PI3K signaling in health and disease, leading to prosurvival signaling in cancer. Neoplastic B cells are enriched for PI3Kδ, which is a target of 3 US Food and Drug Administration (FDA)–approved oral agents in CLL/SLL and FL, namely duvelisib, idelalisib, and copanlisib. Duvelisib also inhibits PI3Kγ (an isoform expressed in T cells, myeloid cells, and others), while copanlisib also targets PI3Kα, an isoform upregulated in certain types of non-Hodgkin lymphoma, along with PI3Kδ.2,3 This spotlight focuses on the newest approved agent, duvelisib (Figure 1).
Targeting PI3K isoforms with duvelisib, idelalisib, and copanlisib in the tumor and the microenvironment. TAM, tumor-associated macrophage.
Targeting PI3K isoforms with duvelisib, idelalisib, and copanlisib in the tumor and the microenvironment. TAM, tumor-associated macrophage.
CLL/SLL cells treated with duvelisib undergo apoptosis under conditions mimicking the lymph node microenvironment (eg, following BCR crosslinking or engagement of CD40).4,5 Exposure to duvelisib decelerates CLL/SLL cell chemotaxis and homing and disrupts the prosurvival PI3K/AKT/mTOR signaling in vitro.4 Furthermore, duvelisib was cytotoxic to multiple B-cell lymphoma cell lines, where it also demonstrated synergy with the BTK inhibitor ibrutinib and the BCL-2 inhibitor venetoclax.6 Interestingly PI3Kγ, which is inhibited by duvelisib, but not by idelalisib, has been shown to have nonredundant pathogenic function in lymphoid cells. Pharmacologic or genetic targeting PI3Kγ reduced CLL/SLL cell migration (and therefore may disrupt CLL cell homing to the protective microenvironment), and duvelisib had a greater effect on migration than single-isoform selective inhibitors.7,8
Studies of duvelisib in R/R CLL/SLL
An initial phase 1 study of duvelisib in R/R CLL/SLL established the maximum tolerated dose of 75 mg twice daily, yet also demonstrated sustained and maximal pharmacodynamic effect at 25 mg twice daily.9 Among 55 R/R CLL/SLL patients, 56% had TP53 mutation and/or deletion 17p (del(17p)), 40% del(11q), and 86.4% unmutated immunoglobulin heavy chain variable gene. The median number of prior therapies was 4 (range, 1-11). The overall response rate (ORR) in R/R CLL/SLL was 56% (n = 31 of 55), with no significant difference between 25 (57.1%, n = 16 of 28) and 75 mg twice daily (54.2%, n = 13 of 24). Responses were primarily partial, with a median progression-free survival (PFS) 15.7 months and median duration of response 21 months. Duvelisib subsequently received approval from the FDA for R/R CLL/SLL after ≥2 lines of prior therapy in 2018 based on the results of the phase 3 DUO study (Table 1).10 Beginning in 2014, DUO enrolled R/R CLL/SLL patients having ≥1 prior therapy. Subjects were randomized 1:1 to duvelisib 25 mg twice daily (n = 160) until progression or unacceptable toxicity or to the anti-CD20 antibody ofatumumab (n = 159). A significant number of patients were enrolled with high-risk disease features such as del(17p) and/or TP53 mutation (31% duvelisib and 33% ofatumumab) and unmutated immunoglobulin heavy chain variable gene (69% duvelisib and 73% ofatumumab). The median number of prior therapies in both arms was 2, and at a median follow-up of 22.4 months, the blinded independent review committee assessment demonstrated a longer median PFS for duvelisib compared with ofatumumab (13.3 vs 9.9 months; hazard ratio, 0.52; P < .0001). Among del(17p) and/or TP53-mutated patients, the median PFS was also higher for duvelisib than ofatumumab (12.7 vs 9.0 months; hazard ratio, 0.40; P = .0002). In addition, the ORR favored duvelisib compared with ofatumumab (73.8% vs 45.3%, P < .0001), with almost all responses being partial (72.5% duvelisib and 44.7% ofatumumab), and at the initial analysis approximately half (49%) of patients receiving duvelisib had received >1 year of therapy. Not surprisingly, the median overall survival (OS) was not reached for either treatment arm with an estimated 12-month OS of 86% for both therapies.
It is important to note that the PFS with duvelisib appears inferior to that with either BTK inhibitors (ibrutinib or acalabrutinib) or the BCL2 inhibitor venetoclax in R/R CLL. Furthermore, the DUO study excluded patients treated with prior BTK or PI3Kδ inhibitors. Meanwhile, few patients had been treated with BTK inhibitors in the phase 1 study of duvelisib (10.9%; n = 6 of 55), and only 1 patient had received idelalisib. This somewhat limits the applicability of the data from these studies in the modern era, where many R/R CLL/SLL patients have been previously treated with novel agents.
Studies of duvelisib in R/R FL
In contrast to CLL, BTK inhibitors demonstrate limited efficacy in FL. In a phase 2 study of ibrutinib in patients with R/R FL (DAWN), ORR was 20.9%, with a median PFS of 4.6 months.12 The PI3K inhibitor landscape in FL includes idelalisib and copanlisib, in addition to duvelisib. All 3 drugs were licensed in the United States for patients with relapsed FL who had received ≥2 prior systemic therapies. While a cross-trial comparison may not be feasible, the clinical efficacy of duvelisib in FL overall appears comparable to that of idelalisib and copanlisib (Table 2).13-15 Duvelisib received FDA approval in FL based on a phase 2 study (DYNAMO) in 2018.13 The study enrolled 129 patients with indolent lymphomas, among them 83 patients with FL who were refractory to both rituximab and a chemotherapy regimen or radioimmunotherapy. In this difficult-to-treat patient population, 94% of patients were refractory to their last therapy, and the median time since completion of the last therapy was short (3.2 months). Duvelisib was administered at a dose of 25 mg twice daily until progression, unacceptable toxicity, or death. ORR (the primary study end point) was 42.2% (1 patient achieved complete remission). Additionally, 85% of patients demonstrated a reduction in lymphadenopathy. Median time to response was 1.9 months. The estimated median duration of response was 10 months, with median PFS of 9.5 months and OS of 28.9 months.13
Early results of frontline activity of duvelisib in combination with an anti-CD20 antibody in FL demonstrated promising activity, with an ORR of ∼90% (CONTEMPO)16 ; however, the study was closed early. Given the adverse events associated with duvelisib (as outlined below), physicians should be cautioned regarding its use in treatment-naive patients. By contrast, FL that relapses early (within 2 years) after initial induction therapy may represent an unmet medical need.17 While idelalisib has been shown to maintain efficacy in this patient population,18 the efficacy of duvelisib in this setting has not been formally analyzed.
Adverse events
As generally seen with other PI3Kδ inhibitors, duvelisib-treated CLL/SLL and FL patients may develop several common all-grade adverse events. The hematologic toxicities include neutropenia (∼30%) and anemia (∼25%). Infection has been a significant adverse event reported with the use of all PI3K inhibitors. Infection occurred in almost 70% of duvelisib-treated patients in the DUO study, where pneumonia was more frequent in duvelisib-treated CLL/SLL patients than in ofatumumab-treated patients (18% vs 6%), as was upper respiratory infection (16% vs 8%). Pneumocystis jirovecii pneumonia (PJP) was also reported in 3 duvelisib-treated patients (2%) who were either not on prophylaxis or intolerant to prescribed prophylaxis. Episodes of Aspergillus and pseudomonal pneumonia were also noted. In this study, fatal adverse events occurred in 19 duvelisib-treated patients (12%), with 4 events being assessed as related to duvelisib, compared with 7 deaths in ofatumumab patients (5%), with infectious events accounting for 11 deaths in duvelisib-treated patients.10 Serious opportunistic infections (bronchopulmonary aspergillosis, cytomegalovirus pneumonia, and PJP) were also noted in the FL DYNAMO study.13 The mechanism of such infectious risk remains unclear, but the frequency of serious infections in patients receiving PI3K inhibitors warrants very careful use of these agents in those with notable history of infections or at higher risk of mortality due to infectious complications. All patients treated with duvelisib should receive PJP prophylaxis. Pre-emptive monitoring for cytomegalovirus reactivation with monthly quantitative cytomegalovirus polymerase chain reaction assay for 6 months, then quarterly, may be considered; however, access to such testing is limited outside of transplant centers.
In the studies of duvelisib, as in those of idelalisib, immune-mediated toxicities such as diarrhea/colitis, hepatitis, pneumonitis, and rash were observed. In both the DYNAMO and DUO trials, diarrhea was the most common adverse event in duvelisib-treated patients, occurring in half of patients (48.8% and 51%, respectively), with ∼15% grade ≥3 in each study. Colitis, reported as a distinct event from diarrhea, was reported in 7.8% and 13%, respectively, with the majority being grade ≥3 colitis (5.4% and 12%, respectively). In the DUO study, corticosteroid therapy was recommended for colitis. Severe hepatitis was relatively rare in both studies, with grade ≥3 transaminitis occuring in 3% of patients.10,13 These immune-mediated toxicities may potentially be explained, at least in part, by the distinct impact of PI3Kδ inhibition in different subsets of T lymphocytes. Regulatory T cells seem particularly sensitive to PI3Kδ inhibition, and depletion of regulatory T cells results in a loss of suppressive regulation on effector T cells.19 While this loss of repression may provide clinical benefit in the form of increased antitumor activity of effector T cells, it may also result in autoimmunity. Colitis, hepatitis, and pneumonitis observed in patients treated with idelalisib all demonstrated increased CD8+ effector T-cell infiltrates in the organ of toxicity. Given the occurrence of these toxicities in idelalisib patients, the pathophysiology of such toxicities may likely be similar with duvelisib. In contrast, copanlisib, which is administered IV and also inhibits PI3Kα, is associated with a distinct adverse event profile, including hypertension and hyperglycemia.15
Overall ∼30% of patients in registration studies of duvelisib discontinued therapy due to treatment-emergent adverse events. Approximately 60% of patients in the DYNAMO study required dose interruption, with ∼20% requiring dose reduction. The most common causes of discontinuation, interruption, or dose reduction of duvelisib have been immune-related adverse events and infection, again underscoring the need for careful monitoring.
Future of duvelisib in FL and CLL/SLL
Treatment with duvelisib results in meaningful activity in FL and CLL/SLL (Tables 1 and 2). Despite the need for caution due to potential toxicities, duvelisib has emerged as an important therapy option for patients with R/R FL and CLL/SLL. Although several other therapies are available for the treatment of R/R FL and CLL/SLL, relapse in both diseases remains a significant problem. Further, toxicities of these alternative therapies may preclude patients with specific comorbidities (ie, atrial fibrillation and concurrent anticoagulation) from being treated with alternative options. While patients with active cardiac disease (defined as unstable angina, myocardial infarction, or unstable arrhythmia within 6 months of study enrollment) were excluded in the DUO and DYNAMO studies, there have not been reported specific cardiac toxicities in duvelisib-treated patients to date. Cautious investigation of duvelisib in patients with cardiac comorbidities may be warranted due to the advanced age of most R/R FL and CLL/SLL patients.
In our opinion, duvelisib has a potential role in the treatment of patients with R/R FL who progress within 2 years after their last chemoimmunotherapy, are poor candidates for it, or have already received multiple chemotherapy regimens. It may serve as an alternative to the lenalidomide-rituximab combination (R2). While the R2 regimen has shown a median PFS of 39.4 months in the AUGMENT study (compared with 9.5 months on DYNAMO), patients were less heavily pretreated, with a median of 1 line of prior therapy (compared with a median of 3 prior lines on DYNAMO), and many were not chemo- or rituximab refractory.20 Duvelisib is a logical choice for R/R FL patients who have progressed on both chemoimmunotherapy and R2 (or are intolerant of the latter).
Duvelisib may be appropriate therapy for patients with relapsed CLL who have history of or active atrial fibrillation, older patients with a cardiac history, or those receiving concurrent anticoagulation, who thus may be poor candidates for BTK inhibitors. Given lack of necessity of intense monitoring for tumor lysis syndrome, duvelisib may be a good alternative to venetoclax in those patients. As the era of chemoimmunotherapy fades in CLL, therapy for relapse after BTK inhibitor and/or BCL-2 antagonists is an area of unmet need. Registrational studies of duvelisib included few such patients, but given its activity in other refractory patients, further investigation in this challenging subset of CLL/SLL patients may be warranted. Given a distinct mechanism of action, one might use duvelisib in patients who relapsed after treatment with venetoclax and who are poor candidates for BTK inhibitors (as above). Additionally, while immunotherapy approaches such as chimeric antigen receptor T-cell therapy or allogeneic transplantation may benefit some R/R CLL/SLL or FL patients, timely delivery of such cell-based therapies can be challenging.21 The activity of duvelisib in both diseases may suggest it as a potentially useful option while delivery of cell therapies is being considered.
Meanwhile, strategies to enhance efficacy and improve toxicity of duvelisib are now being pursued in trials. These include combination studies and alternative dosing regimens. In an ongoing phase 1 clinical trial of duvelisib in combination with venetoclax in patients with R/R CLL (NCT03534323), treatment may be stopped in those patients who achieve minimal residual disease negativity in the bone marrow after 1 year of therapy. A phase 2 study of intermittent dosing of duvelisib in patients with R/R CLL (NCT03961672), where duvelisib is administered at standard dosing during a 3-month induction followed by twice weekly maintenance, aims to mitigate concerns regarding toxicities of continuous duvelisib monotherapy.
PI3K inhibition remains a subject of active investigation in lymphoid malignancies. Umbralisib, a dual PI3K/casein kinase 1ε inhibitor, has demonstrated efficacy across multiple non-Hodgkin lymphoma subtypes.22 Clinical trials with the selective PI3Kδ inhibitors ME-401 and INCB040093 are ongoing. However, to fully realize the potential of PI3Kδ inhibitors in CLL and FL, we propose that the following questions should be answered: (1) What is the activity of PI3K inhibitors in patients with CLL who progress on BTK inhibitors and/or venetoclax? (2) How do we best mitigate the adverse events associated with inhibition of the p110δ isoform? (3) Can use of PI3K inhibitors in earlier lines of therapy enhance efficacy of other targeted agents and thus help attain deeper responses? We believe that the efficacy and safety profile of duvelisib reported to date provides an appropriate foundation to help further explore these questions in future trials and supports the current use of duvelisib monotherapy in select patients with R/R FL and CLL/SLL.
Acknowledgment
A.V.D. is a Leukemia & Lymphoma Society Scholar in Clinical Research.
Authorship
Contribution: K.P., A.V.D., and J.M.P. each wrote the review, and each author reviewed and critiqued the manuscript.
Conflict-of-interest disclosure: K.P. has been a consultant for Astra Zeneca, Pharmacyclics, Janssen, Genentech, Celgene, Sunesis, and Verastem. A.V.D. has been a consultant for Astra Zeneca, Abbvie, Teva Oncology, Verastem, Gilead Sciences, Genentech, TG Therapeutics, Celgene, Curis, Janssen, Pharmacyclics, and Seattle Genetics and has received research funding from Aptose Biosciences, Verastem, Astra Zeneca, Gilead Sciences, Takeda, Genentech, Bayer, and Bristol-Meyers Squibb. J.M.P. has been a consultant for Gilead Sciences, Pharmacyclics, TG Therapeutics, and Astra Zeneca.
Correspondence: John M. Pagel, Swedish Cancer Institute, 1221 Madison St, Seattle, WA 98104; e-mail: john.pagel@swedish.org.