In this issue of Blood, Hu et al elegantly demonstrate that steroid receptor coactivator-3 (SRC-3) is essential for maintenance of hematopoietic stem cell (HSC) homeostasis via repression of mitochondrial biogenesis by acetyltransferase GCN5-mediated posttranslational modification of peroxisome proliferator–activated receptor γ coactivator 1-α (PGC-1α).1
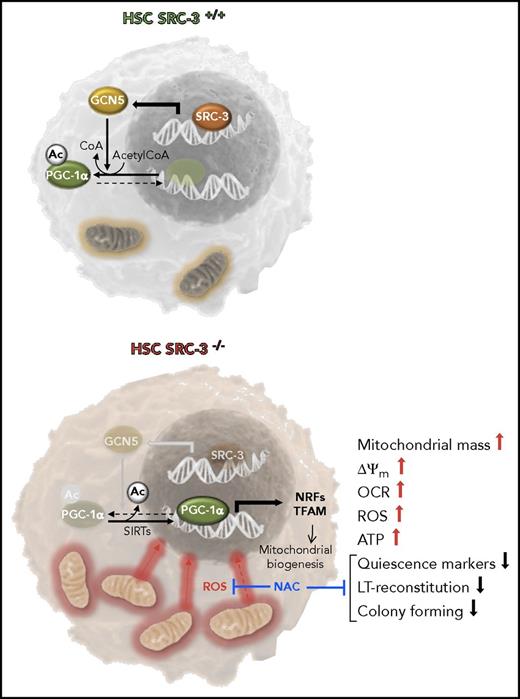
Function of the SRC-3 pathway in HSCs. SRC-3 is shown bound to the promoter region of the gene coding the acetyltransferase GCN5 in WT HSCs. CGN5 contributes to acetylate PGC-1α, thus inhibiting its cotranscriptional activity. This results in repression of the mitochondrial oxidative metabolism and maintenance of the quiescent state in HSCs. In SRC-3−/− HSCs, the deacetylation state of PGC-1α is shown to prevail because of the noncounteracted activity of sirtuins (SIRTs). In this state, PGC-1α can exert its transcriptional activity upregulating the expression of genes involved in mitochondrial biogenesis. This results in increased mass of mitochondria and mtDNA copy number, higher ΔΨm, and enhanced OCR, ROS generation, and ATP production. The phenotype of SRC-3−/− HSCs is characterized by loss of quiescence and lower long-term (LT) reconstitution capacity. These latter features in SRC-3−/− HSCs are prevented by treatment with the antioxidant N-acetyl cysteine (NAC) suggesting the involvement of ROS-mediated signaling. NRFs, nuclear respiratory factors; TFAM, mitochondrial transcription factor A.
Function of the SRC-3 pathway in HSCs. SRC-3 is shown bound to the promoter region of the gene coding the acetyltransferase GCN5 in WT HSCs. CGN5 contributes to acetylate PGC-1α, thus inhibiting its cotranscriptional activity. This results in repression of the mitochondrial oxidative metabolism and maintenance of the quiescent state in HSCs. In SRC-3−/− HSCs, the deacetylation state of PGC-1α is shown to prevail because of the noncounteracted activity of sirtuins (SIRTs). In this state, PGC-1α can exert its transcriptional activity upregulating the expression of genes involved in mitochondrial biogenesis. This results in increased mass of mitochondria and mtDNA copy number, higher ΔΨm, and enhanced OCR, ROS generation, and ATP production. The phenotype of SRC-3−/− HSCs is characterized by loss of quiescence and lower long-term (LT) reconstitution capacity. These latter features in SRC-3−/− HSCs are prevented by treatment with the antioxidant N-acetyl cysteine (NAC) suggesting the involvement of ROS-mediated signaling. NRFs, nuclear respiratory factors; TFAM, mitochondrial transcription factor A.
The metabolic phenotype of a cell is emerging as a critical determinant of the fate of stem cells. This is also true for HSCs with a number of players in the metabolic rewiring of HSCs already described.2 Hu et al add a new piece of evidence identifying in SRC-3/GCN5/PGC-1α, a hitherto unappreciated pathway in the maintenance of the HSCs quiescence, which regulates mitochondrial metabolism. SRC-3 is a steroid receptor transcriptional coactivator involved in a number of processes including cell growth and proliferation in normal and cancer cells.3 In particular, SRC-3 has proved to be critical for the maintenance of the cancer stemlike cells and normal hematopoiesis. SRC-3 was found to be highly expressed in murine hematopoietic Lin−Sca1+c-Kit+ (LSK) cells (enriched in HSCs) as compared with lineage-restricted progenitor cells. Hence, Hu et al carried out an extensive characterization of the hematopoietic compartment in SRC-3 knockout mice. Comparing the SRC-3−/− with wild-type (WT) mice, the following was found: (1) increased LSKs and long-term reconstitution HSCs (LT-HSCs), (2) altered cell cycle state in LT-HSCs indicative of reduced quiescence, (3) increased mobilization of LSKs, (4) increased sensitivity of HSCs to cytotoxic stress and irradiation, and (5) reduced reconstitution capacity of HSCs in noncompetitive and competitive bone marrow transplantation assays and impairment of in vitro colony-forming capacity. Importantly, bone marrow of lethally irradiated SRC-3−/− mice normally reconstituted WT HSCs showing that SRC-3 intrinsically regulates HSC function.
Next, Hu et al performed a differential microarray gene expression analysis identifying upregulation of genes linked to the mitochondrial oxidative phosphorylation (OXPHOS) in SRC-3−/− LSK cells. Thus, in the second part of their study Hu et al validated this observation demonstrating the following in SRC-3−/− HSCs: (1) augmented mitochondrial mass and increased mitochondrial DNA (mtDNA) copy number; (2) enhanced expression of mitochondrial markers; (3) higher mitochondrial membrane potential (ΔΨm), oxygen consumption rates (OCRs), and reactive oxygen species (ROS) generation; and (4) enhanced glucose uptake and adenosine triphosphate (ATP) production (see figure). Altogether, the above observations are consistent with a metabolic rewiring in SRC-3−/− HSCs from an aerobic glycolysis to a mitochondria-driven OXPHOS phenotype.
PGC-1α is a master regulator of mitochondrial biogenesis,4 and consistent with this notion, Hu et al found enhanced expression of PGC-1α target genes. However, the expression of PGC-1α was unchanged in SRC-3−/− as compared with WT HSCs suggesting alteration at the posttranslational level. Indeed, PCG-1α is known to be regulated by different reversible covalent modification including inhibitory acetylation at a specific lysine residue.
Accordingly, the acetyltransferase GCN5, known to target PGC-1α,5 was found to be downregulated in SRC-3−/− HSCs, and PGC-1α hypoacetylated. SRC-3 can directly control the expression of GCN5 by specifically binding to its promoter region.3 Notably, overexpression of GCN5 in SRC-3−/− HSCs (1) enhanced acetylation of PGC-1α, (2) reduced mitochondrial mass and membrane potential, (3) decreased ROS production, and (4) caused recovery of quiescence and long-term repopulating capacity.
Taken together, the findings of Hu et al corroborate the notion that maintenance of HSC quiescence and self-renewal capacity requires suppression of the mitochondrial OXPHOS. This is partly accomplished by the highly hypoxic bone marrow microenvironment where HSCs reside that forces them to rely on a glycolysis-driven low-ROS-producing metabolism.6,7 Activation of the mitochondrial oxidative metabolism thus appears to be a prerequisite to the egress of HSCs from their quiescent state, priming them to proliferate and likely making them more responsive to specific differentiation hits.
The novel function of SRC-3 needs now to be integrated with that of other players, such as mTOR, LKB1, AMPK, and FoxOs,1,7 just to name a few, in controlling the balance between quiescent and activated state in the HSC compartment. Notably, all these factors converge, though by different mechanisms, in the control of the mitochondrial biogenesis. Other pathways have been reported to control HSC state/fate by modulating the mitochondrial dynamics (ie, fusion vs fission) and organelle quality control (ie, mitophagy).8
In keeping with the multifaceted physiology of mitochondria, what further remains to be established is(are) the mechanism(s) that these amazing organelles put in action in reprogramming the HSCs compartment. In addition to the more efficient bioenergetic capacity of the OXPHOS, needed to cope with an enhanced demand for biosynthetic processes, mitochondria are essential in controlling redox and calcium balance, as well as cell death. Therefore, they are at the interface between environmental cues and the control of the epigenetic identity. Indeed, mitochondria export intermediate metabolites needed for the activities of chromatin remodeling enzymes such as histone acetylases/deacetylases and DNA and histone methylases/demethylases.9
Furthermore, the role of mitochondrial ROS-mediated signaling must not be neglected in this context. ROS, once considered a harmful by-product of cellular respiration, is now recognized as a powerful second messenger, proven to activate/inactivate protein phosphatases and kinases as well as transcription factors, when kept below a cytotoxic threshold.10 Insightfully, Hu et al showed that treatment of SRC-3−/− mice with the antioxidant NAC resulted, under conditions suppressing the overproduction of ROS in HSCs, in rescue of the expanded number, reduced quiescence, and increased proliferation of SRC-3−/− LT-HSCs and in improvement of their engraftment. This finding is in line with previously reported observations showing that antioxidant treatment prevented/reversed alterations of the HSCs homeostasis elicited by knocking out genes coding for mTOR, ATM, FoxO3, LKB1, paving the way to new as well as challenging investigations. Indeed, the chemical nature of the reactive oxygen (and possibly nitrogen) species acting as signaling molecules and their target(s) remain still largely elusive in the context of the HSC biology. Nevertheless, disentangling these aspects might provide effective clinical tools in regenerative medicine. Finally, the study by Hu et al stimulates more in-depth examinations of the role of SRC-3 in cancer stem cells and tumor genesis, given its overexpression in different human cancers, representing an exciting avenue for future exploration.
Conflict-of-interest disclosure: The authors declare no competing financial interests.