Key Points
CBT after FLU-CY-ATG-2-Gy TBI with at least 4 × 107 frozen NCs per kilogram leads to satisfactory OS in refractory SAA.
CBT is a valuable curative option in young patients with refractory idiopathic SAA and no available matched unrelated donors.
Abstract
Outcomes remain poor for refractory severe aplastic anemia (SAA) patients. Alternative donor transplantation may be considered, but results from previous studies are not encouraging. We conducted a prospective nationwide phase 2 study to assess unrelated cord blood (CB) transplantation (CBT) efficacy and safety in refractory SAA patients (Aplastic Anemia and Cord Blood Transplantation protocol). To demonstrate a significant difference in 1-year survival from 20% (null hypothesis) to 50% (alternative hypothesis), we needed to include 25 transplanted patients and therefore included 26 (median age, 16 years). Eligibility criteria required 1 or 2 unrelated CB units, containing separately or together >4 × 107 frozen nucleated cells (NCs) per kilogram of recipient body weight. Conditioning regimen comprised fludarabine (FLU), cyclophosphamide (CY), antithymocyte globulin (ATG), and 2-Gy total body irradiation (TBI). With a median follow-up of 38.8 months, engraftment occurred in 23 patients (88%); cumulative incidences of grade II-IV acute and chronic graft-versus-host disease were 45.8% and 36%, respectively. Twenty-three patients were alive at 1 year, with an 88.5% overall survival (OS) rate, differing significantly from the expected 20% (P < .0001; 84% OS at 2 years). CBT with units containing ≥4 × 107 frozen NCs per kilogram is therefore a valuable curative option for young adults with refractory SAA and no available matched unrelated donors. This trial was registered at www.clinicaltrials.gov as #NCT01343953.
Medscape Continuing Medical Education online

In support of improving patient care, this activity has been planned and implemented by Medscape, LLC and the American Society of Hematology. Medscape, LLC is jointly accredited by the Accreditation Council for Continuing Medical Education (ACCME), the Accreditation Council for Pharmacy Education (ACPE), and the American Nurses Credentialing Center (ANCC), to provide continuing education for the healthcare team.
Medscape, LLC designates this Journal-based CME activity for a maximum of 1.00 AMA PRA Category 1 Credit(s)™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
All other clinicians completing this activity will be issued a certificate of participation. To participate in this journal CME activity: (1) review the learning objectives and author disclosures; (2) study the education content; (3) take the post-test with a 75% minimum passing score and complete the evaluation at http://www.medscape.org/journal/blood; and (4) view/print the certificate. For the CME questions, see page 768.
Disclosures
CME questions author Laurie Barclay, freelance writer and reviewer, Medscape, LLC, owns stock, stock options, or bonds from Pfizer. Associate Editor Robert Zeiser and the authors declare no competing financial interests.
Learning objectives
Upon completion of this activity, participants will be able to:
Describe the regimen used for unrelated cord blood transfusion (CBT) and rates of graft-versus-host disease in patients with refractory severe aplastic anemia (SAA), based on a prospective nationwide phase 2 study
Interpret engraftment rates associated with unrelated CBT in patients with refractory SAA
Determine the safety and overall survival of CBT in patients with refractory SAA
Release date: August 16, 2018; Expiration date: August 16, 2019
Introduction
Outcomes remain poor for severe aplastic anemia (SAA) patients refractory to first-line immunosuppressive therapy (IST) without matched unrelated donors (MUDs). Recent use of eltrombopag shows blood count improvements (40% of cases),1-3 but most refractory patients remain unresponsive to eltrombopag and other second-line treatments, exposing them to life-threatening complications. Moreover, clonal evolution, including paroxysmal nocturnal hemoglobinuria, myelodysplastic syndrome (MDS), and acute myeloid leukemia, may eventually occur.4 MDS and acute myeloid leukemia complications are more frequent in refractory SAA patients,5 whose prognoses are usually grim.4 Hematopoietic stem cell transplantation (HSCT) using alternative donor sources (mismatched unrelated donors, cord blood, and haploidentical family donors) may be curative in these cases, despite risks including graft rejection, infectious complications, and much higher rates of graft-versus-host disease (GVHD) than in transplantations from matched related donors or MUDs.6,7 In this report, we assessed the efficacy and safety of unrelated cord blood (CB) transplantation (CBT) in refractory SAA patients (Aplastic Anemia and Cord Blood Transplantation [APCORD] protocol).
Study design
Patients with primary refractory SAA at 6 months after first-line IST with antithymocyte globulin (ATG) and cyclosporine (CSA), or in relapse and refractory to a second course of ATG plus CSA without an available MUD, were eligible if 1 or 2 unrelated CB units were available (containing separately or together >4 × 107 frozen nucleated cells [NCs] per kilogram and no more than 2 in 6 HLA mismatches between each CB unit and the patient). CB unit selection required negative donor-specific anti-HLA antibodies. Patients with isolated bone marrow cytogenetic abnormalities (no morphologic evidence of clonal evolution) were also eligible. The Saint Louis Hospital Institutional Review Board and French authorities approved the protocol. All patients provided written informed consent as per the Helsinki Declaration.
The conditioning regimen comprised fludarabine (FLU) 30 mg/m2 per day (day −6 to day −3), cyclophosphamide (CY) 30 mg/kg per day (day −6 to day −3), ATG 2.5 mg/kg per day at day −3 and day −2 (5 mg/kg total dose), and total body irradiation (TBI) (2 Gy, day −2). All patients received 1 injection of an anti-CD20 monoclonal antibody (rituximab) (150 mg/m2) to prevent Epstein-Barr virus (EBV) reactivation (day +5).8 CSA alone was given as GVHD prophylaxis, targeting trough concentrations 200 to 400 ng/mL for 3 months before progressive tapering to stop at 1 year. The primary end point was overall survival (OS) at 1 year. Secondary end points included cumulative incidences of engraftment, acute and chronic GVHD (aGVHD9 and cGVHD10 ), infections, relapse, and causes of death. When this protocol was designed, Japanese and European studies using CB in SAA showed 2-year OS of 41% and 3-year OS of 38%, respectively.11,12 Our study used the Fleming Single-Stage Phase 2 Design, with a sample size computed to demonstrate (exact binomial test) a significant improvement in OS at 1 year from 20% (null hypothesis, treatment considered unacceptable) to 50% (alternative hypothesis, minimal OS rate to consider treatment as a valuable option for patients), with type I and type II error rates set at 0.05. To indicate treatment efficacy, a minimum sample of 25 transplanted patients was required, with at least 9 patients alive at 1 year. Full analysis was based on all patients undergoing transplantation. Secondary sensitivity analysis comprised an intent-to-transplant population of all 27 included patients. OS was estimated using the Kaplan-Meier method, although cumulative incidence curves used nonparametric estimators with death as a competing event. Data were analyzed using R 3.3.2 software.
Results and discussion
Twenty-nine consecutive patients were included between June 2011 and October 2015. One patient was diagnosed with dyskeratosis congenita, and a MUD was identified in another patient between inclusion and the anticipated CBT date (exclusion criteria). Another patient died of septic shock before CBT (day −4 of the conditioning regimen). Analysis was therefore limited to the remaining 26 patients. Median age at CBT was 16 years (interquartile range [IQR], 9.3-23.4). Table 1 summarizes patient and cord blood characteristics. All patients received at least 1 course of IST before transplantation (2 courses, n = 11). Long waits from diagnosis to transplantation (median, 12 months; IQR, 8.7-17.8) and heavily transfused statuses reflect the disease’s refractoriness (Table 1).
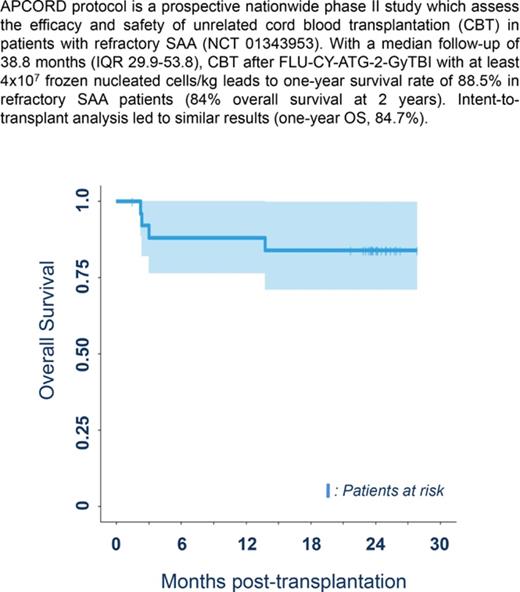
Median follow-up was 38.8 months (IQR, 29.9-53.8), which is long enough for our results to be considered to be robust.13 Three patients died before 1 year due to infections arising from nonengraftment (n = 2) and GVHD (n = 1), with a 1-year survival rate of 88.5% (95% confidence interval [CI], 69.8%-97.6%), thereby achieving the study’s primary objective (significant difference from the expected 20%; P < .0001). Intent-to-transplant analysis led to similar results (1-year OS, 84.7%; 95% CI, 72.0%-99.7%; P < .0001). One-year treatment-related mortality was 11.5% (95% CI, 2.4-30.2). A further patient died of severe cGVHD at 13.9 months, leading to a 2-year survival rate of 84.6% (95% CI, 71-100) (Figure 1A).
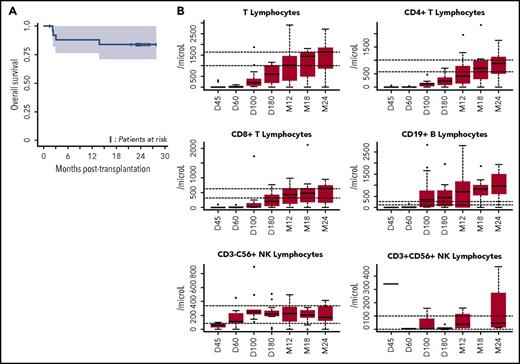
OS and immune reconstitution after CBT in patients with refractory aplastic anemia. (A) OS after CBT in patients with refractory aplastic anemia. Estimated 1-year OS of 88.5% (95% CI, 77-100) and 2-year OS of 84% (95% CI, 71-100 are represented, Kaplan-Meier curve). OS was similar between patients who received 1 or 2 unrelated CB units (as was the case for cumulative incidences of engraftment, aGVHD and cGVHD, and infections). (B) Reconstitution of T-, B-, and natural killer (NK)-cell subsets. All patients were systematically monitored posttransplantation for immune reconstitution at days +45 and +60, and months +3, +6, +12, and +24. T lymphocytes, CD4, CD8, B, NK lymphocytes were prospectively measured. For each time point, 25th to 75th percentiles are shown, along with the median.
OS and immune reconstitution after CBT in patients with refractory aplastic anemia. (A) OS after CBT in patients with refractory aplastic anemia. Estimated 1-year OS of 88.5% (95% CI, 77-100) and 2-year OS of 84% (95% CI, 71-100 are represented, Kaplan-Meier curve). OS was similar between patients who received 1 or 2 unrelated CB units (as was the case for cumulative incidences of engraftment, aGVHD and cGVHD, and infections). (B) Reconstitution of T-, B-, and natural killer (NK)-cell subsets. All patients were systematically monitored posttransplantation for immune reconstitution at days +45 and +60, and months +3, +6, +12, and +24. T lymphocytes, CD4, CD8, B, NK lymphocytes were prospectively measured. For each time point, 25th to 75th percentiles are shown, along with the median.
In this particular high-risk population, only 3 of the 26 patients failed to engraft with no clear explanation (1 death at day +71, another at day +92 after a second haploidentical HSCT, although a third patient was still alive 31 months after a second haploidentical HSCT), resulting in a 60-day cumulative incidence of full chimerism of 88% (95% CI, 75-100). Engraftment has previously been a major barrier to CBT in refractory AA patients, mainly due to transfusion-induced allogeneic immunization.11,12 Retrospective studies suggested a pivotal role of NC doses (>3.9 × 107/kg)12 along with the use of a fludarabine-/TBI-based regimen for engraftment and OS.11,12 The APCORD protocol built on these retrospective findings and prospectively confirms the benefit of controlling for these factors.
The second limitation of using CBT in SAA has been unacceptable rates of severe infection.11,12 Previous series reported a high rate of severe or fatal infections, associated with delayed immune reconstitution.14,15 In APCORD, 8 patients experienced a total of 18 grade 3 infections (6-month cumulative incidence of 51.1%; 95% CI, 34-68.1). This lower infection rate, compared with previous observations in similar settings, may arise from overall improved immune reconstitution,16 possibly due to the high NC dose (Figure 1B). Moreover, none of our patients developed EBV-associated lymphoproliferative disease, thereby illustrating the efficacy of an early anti-CD20 injection (at day +5)8 and EBV DNA monitoring, with early preemptive use of anti-CD20 in patients with increasing viral load.17
One main reason for maintaining the CBT program in AA patients was the low incidence of GVHD after CBT, despite HLA mismatches.11,12 Ten patients developed grade 2-4 aGVHD (100-day cumulative incidence; 45.8% [95% CI, 25.3-66.4]). Nine patients developed cGVHD, including 5 following aGVHD and 4 de novo (1-year cumulative incidence; 36% [95% CI, 16.6-55.4]). Overall, 18 of the 22 patients alive had stopped active immunosuppression (median time, 18 months posttransplantation) at last follow-up. However, 3 patients presented extensive cGVHD, although 1 of the 3 deaths within 1 year was GVHD-related. These results justify using a minimal ATG dose (5 mg/kg total dose) in APCORD, which may also lower nonengraftment rates.
Three of our patients had isolated bone marrow clonal cytogenetic abnormalities at transplantation (monosomy 7 and trisomy 8, with no morphologic evidence of MDS). Due to unacceptable rates of toxicity in myeloablative-conditioning regimens involving CBT and aplastic anemia,11 we decided to include these patients in this study: all 3 are doing well at 18.4, 23.9, and 24.8 months post-CBT, with no clonal cytogenetic abnormalities.
In conclusion, CBT with units containing at least 4 × 107 frozen NCs per kilogram after a FLU-CY-TBI-ATG conditioning regimen showed very encouraging results in this particular high-risk population. Pediatric and young adult SAA patients refractory to first-line IST may therefore now be safely considered for CBT.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank colleagues who took part in the data and safety board and monitored the study. André Tichelli (University Hospital of Basel, Switzerland) acted as president of the data safety monitoring board (DSMB), assisted by Nadine Petitpain (Brabois Hospital, Nancy, France), Michel Duval (Sainte-Justine University Hospital, Montréal, Canada), and Jacob Passweg (University Hospital of Basel, Switzerland). The authors also thank the patients and their families for kindly accepting to take part in this research, as well as the patient advocacy groups HPN France-Aplasie médullaire and HPN Solidarité Catalane for support.
This work was supported by an unrestricted grant from Amgen Pharmaceuticals dedicated to data monitoring.
Authorship
Contribution: R.P.d.L., S.C., E.G., and G.S. conceived and designed the study; R.P.d.L., S.C., C.J., A. Sirvent, C.G., A.R., V.G., J.C., F.R., J.-H.D., E.F., B.B., C.P., P.S.R., A. Salmon, S.F., F.S.d.F., M.T.R., J.-O.B., M.M., J.L., E.G., and G.S. provided study materials and patients and gave final approval of the manuscript; R.P.d.L., S.C., and G.S. collected, assembled, analyzed, and interpreted data and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
A complete list of the members of the Francophone Society of Bone Marrow Transplantation and Cellular Therapy appears in the online appendix.
Correspondence: Régis Peffault de Latour, Service d’Hématologie Greffe, Centre de Reference Aplasie Médullaire et HPN, Hôpital Saint-Louis, 1 Ave Claude Vellefaux, Paris, France; e-mail: regis.peffaultdelatour@aphp.fr.