Key Points
Duvelisib significantly improved progression-free survival and overall response rates compared with ofatumumab in RR CLL/SLL patients.
Duvelisib’s efficacy and manageable safety profile support its consideration as a novel, oral monotherapy for RR CLL/SLL patients.
Abstract
Duvelisib (also known as IPI-145) is an oral, dual inhibitor of phosphatidylinositol 3-kinase δ and γ (PI3K-δ,γ) being developed for treatment of hematologic malignancies. PI3K-δ,γ signaling can promote B-cell proliferation and survival in clonal B-cell malignancies, such as chronic lymphocytic leukemia (CLL)/small lymphocytic lymphoma (SLL). In a phase 1 study, duvelisib showed clinically meaningful activity and acceptable safety in CLL/SLL patients. We report here the results of DUO, a global phase 3 randomized study of duvelisib vs ofatumumab monotherapy for patients with relapsed or refractory (RR) CLL/SLL. Patients were randomized 1:1 to oral duvelisib 25 mg twice daily (n = 160) or ofatumumab IV (n = 159). The study met the primary study end point by significantly improving progression-free survival per independent review committee assessment compared with ofatumumab for all patients (median, 13.3 months vs 9.9 months; hazard ratio [HR] = 0.52; P < .0001), including those with high-risk chromosome 17p13.1 deletions [del(17p)] and/or TP53 mutations (HR = 0.40; P = .0002). The overall response rate was significantly higher with duvelisib (74% vs 45%; P < .0001) regardless of del(17p) status. The most common adverse events were diarrhea, neutropenia, pyrexia, nausea, anemia, and cough on the duvelisib arm, and neutropenia and infusion reactions on the ofatumumab arm. The DUO trial data support duvelisib as a potentially effective treatment option for patients with RR CLL/SLL. This trial was registered at www.clinicaltrials.gov as #NCT02004522.
Introduction
Chronic lymphocytic leukemia (CLL) is the most common adult lymphoproliferative disorder in Western countries, with >20 000 estimated new cases in the United States in 2017 and >4600 deaths.1 The timing and selection of therapy is largely informed by multiple clinical and biological factors, specifically disease stage, presence of poor prognostic molecular features such as 17p13.1 deletions [del(17p)] and TP53 mutations, and the general status of individual patients, many of whom are elderly with comorbid conditions precluding aggressive chemoimmunotherapy.2,3 Chemotherapy, specifically alkylating agents and purine analogs, and monoclonal CD20 antibody therapy have long represented the traditional backbone of frontline therapy.4 The comparator agent for the DUO trial, ofatumumab, is a humanized anti-CD20 antibody with single-agent efficacy against refractory CLL and a US Food and Drug Administration–approved treatment option.5,6 Recently approved therapies targeting key pathways of CLL cell proliferation and survival, particularly Bruton tyrosine kinase (BTK), phosphatidylinositol 3-kinase δ (PI3K-δ), and BCL2, have greatly improved outcomes for CLL patients and have been integrated into the CLL treatment landscape.7 However, except for allogeneic stem cell transplantation, there is general consensus that current therapies for CLL/small lymphocytic lymphoma (SLL) are not curative, and most patients will ultimately die of their disease.8,9 Given the inevitable development of resistance or intolerance to available therapies, there remains an urgent need to develop novel effective and tolerable CLL/SLL treatment options.
Duvelisib is an oral dual PI3K-δ,γ inhibitor that targets key signaling pathways that promote the growth and survival of hematologic malignancies. PI3K-δ inhibition blocks the survival and proliferation of malignant B cells,10-12 whereas PI3K-γ inhibition disrupts the recruitment and differentiation of T cells and macrophages within the tumor microenvironment that support malignant B-cell maintenance.13-16 Dual PI3K-δ,γ inhibition has shown greater activity in preclinical models of CLL than blocking either isoform alone.17-21 Accordingly, duvelisib’s unique therapeutic potential offers a novel approach to treat patients with CLL/SLL.
The efficacy and safety profile of duvelisib monotherapy for the treatment of hematologic malignancies was demonstrated in a phase 1 trial.22,23 Clinically meaningful activity was observed in a cohort of relapsed or refractory (RR) CLL/SLL patients with a 56% overall response rate (ORR) and a median progression-free survival (PFS) of 15.7 months.22,24 Reductions in serine/threonine protein kinase B phosphorylation (p-AKT) and proliferating (Ki67+) CLL cells were seen in CLL patient samples following duvelisib administration, demonstrating pharmacodynamic evidence of PI3K inhibition. Based on the combined efficacy, safety, pharmacokinetics, and pharmacodynamic data from this phase 1 study, duvelisib 25 mg twice daily was selected as a clinically active dose for a phase 3 investigation in CLL/SLL.22
Building on these encouraging phase 1 results, the global phase 3 randomized DUO study was initiated in 2014 to fully characterize the efficacy and safety of duvelisib monotherapy in patients with RR CLL/SLL when compared with an approved standard of care, ofatumumab monotherapy. Herein are reported the final analysis results for this trial.
Methods
Study design and treatment
DUO is a global, multicenter, randomized, open-label, phase 3 trial comparing the efficacy and safety of duvelisib monotherapy with ofatumumab monotherapy for RR CLL/SLL. Between 21 January 2014 and 9 December 2015, 319 patients with RR CLL/SLL were randomized 1:1 to study treatment with duvelisib (n = 160) or ofatumumab (n = 159) at 62 clinical study sites in 11 countries. Patients were required to have active CLL/SLL disease necessitating treatment, per the International Workshop on CLL25 criteria or Revised International Working Group26 criteria that had progressed during or relapsed after at least 1 previous CLL/SLL therapy. Radiologically measurable disease, defined as at least 1 lymph node or tumor mass measuring >1.5 cm by computed tomography scan, was required. Other standard eligibility requirements included adequate renal and hepatic function, hemoglobin ≥8.0 g/dL, and platelet count ≥10 000 µL with or without transfusion support. There was no eligibility requirement with regard to neutrophil count. Key exclusion criteria included prior treatment with BTK or PI3K-δ inhibitors; refractoriness to prior ofatumumab therapy; or a history of Richter transformation, prolymphocytic leukemia, or allogenic stem cell transplant. Pneumocystis jirovecii prophylaxis concomitant with study drug treatment was required for all patients on both treatment arms. Per protocol, antiviral prophylaxis was recommended to be implemented at the discretion of the treating investigator.
Patient stratification at randomization included the presence or absence of del(17p), grade 4 cytopenia, and refractoriness/early relapse to purine analog based therapy (defined as progression <12 months after fludarabine/pentostatin). Patients randomized to the duvelisib arm self-administered 25 mg capsules twice daily continuously in 28-day cycles except for the first cycle (21 days) to align with administration of ofatumumab infusions. Patients were allowed to take duvelisib for up to 18 cycles, until disease progression, or until unacceptable toxicity. Additional treatment with duvelisib beyond 18 cycles was allowed based on the judgement of the investigator. Guidance for adverse event (AE) management was provided in the protocol and included dose modifications (interruptions and reductions) for treatment-related grade 2 or higher pneumonitis or pneumonia, grade 3 or higher nonhematologic AEs, febrile neutropenia, and thrombocytopenia with hemorrhage. Corticosteroids were recommended for colitis or persistent or recurrent severe diarrhea and in combination with antibiotic therapy in patients presenting with pulmonary symptoms or radiographic changes suspicious for pneumonitis.
Ofatumumab arm dosing was based on the dose and schedule outlined in the approved product labeling for monotherapy in relapsed CLL at the time the study was initiated and could not exceed the 12 doses (within 7 cycles) as described in the prescribing information.27
Study visits for duvelisib arm patients occurred on cycle 1 day 1 and 8, cycle 2 day 1 and 15, and day 1 of every other cycle thereafter. Study visits for ofatumumab arm patients were similar except for additional visits on cycle 1 day 15 and cycle 2 day 8 and 22, for study drug infusions. Patients on either treatment arm that experienced radiographically confirmed progressive disease had the option to cross over to a separate extension study to receive the opposite therapy.
Study end points and assessments
The primary end point was PFS, defined as time from randomization to first documentation of progressive disease as determined by an independent review committee (IRC) or death attributable to any cause. Key secondary end points included the following: (1) ORR, with overall response (per IRC determination) defined as the best response of complete response/remission (CR), CR with incomplete marrow recovery, partial response/remission (PR), or PR with lymphocytosis (PRwL), according to the International Workshop on CLL25,26 or Revised International Working Group response criteria,28 with modification for treatment-related lymphocytosis; and (2) overall survival (OS). Per protocol, a response of PRwL was limited to patients with lymphadenopathy as the only abnormal baseline group A criterion (eg, no organomegaly and normal blood lymphocyte count <4000/µL) who achieved a PR (≥50% reduction in lymphadenopathy) but had postbaseline isolated progressive lymphocytosis. Response assessments for both arms included complete blood count with differential, computed tomography scan, disease-associated symptom review, and bone marrow biopsy (for CR confirmation only) that were performed after every 2 cycles until cycle 7, every 4 cycles until cycle 19, and then every 6 cycles until disease progression, start of new anticancer therapy, or subject withdrawal.
Efficacy analysis and statistics
PFS was compared between the duvelisib and ofatumumab treatment arms using a stratified log-rank test (1-sided) with the overall 1-sided significance level controlled at .025. The hazard ratio (HR) for duvelisib/ofatumumab and the corresponding 2-sided 95% confidence interval (CI) was estimated using a stratified Cox proportional hazards model, and PFS for both arms was plotted using the Kaplan-Meier method. The ORR was analyzed using the Cochran-Mantel-Haenszel test to compare treatments. OS (time from randomization to death) was compared between treatment arms using a stratified 1-sided log-rank test, and the HR along with the 95% CI was estimated using a stratified Cox model. Kaplan-Meier plots for OS were generated for each treatment group. The lymph node response rates (≥50% reduction in target lymph nodes from baseline) between treatment arms was analyzed using the 1-sided Cochran-Mantel-Haenszel test.
Safety methods
AEs and hematology and blood chemistry laboratory values were assessed at each study visit or as clinically warranted and coded using MedDRA version 16.1. The severity of AEs and applicable laboratory values was graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events (NCI-CTCAE) version 4.03.
Data sharing statement
For original data, please contact nmirza@verastem.com.
Results
Patient baseline characteristics and disposition
The baseline characteristics and stratification of study patients were well balanced between the 2 treatment arms (Table 1). Patients were predominantly male (60%) with a median age in both arms of 69 years. Most patients had a baseline ECOG PS of 0 or 1, with a similar percentage of patients with an ECOG PS of 2 (duvelisib 7%; ofatumumab 10%). The median time from initial diagnosis was 7.5 years for duvelisib and 6.7 years for ofatumumab, and 56% of patients in both arms were greater than or equal to Rai stage III at the start of therapy. Nearly half of duvelisib- and ofatumumab-treated patients had bulky disease (46% and 45%, respectively), and both arms had similar median baseline lymphocyte counts (38 × 109/L and 35 × 109/L). Central laboratory determination of molecular features identified del(17p) and/or TP53 mutation in approximately one-third of patients (duvelisib 31%; ofatumumab 33%) and unmutated IGHV status in 69% and 73% of duvelisib and ofatumumab patients, respectively.
On both study arms, the median number of prior therapies was 2, with approximately one-third having received ≥3 prior lines of therapy (supplemental Table 1; available on the Blood Web site). Most patients had previously received an alkylating agent (93% duvelisib; 95% ofatumumab), a monoclonal antibody (78% duvelisib; 83% ofatumumab), and purine analog (60% duvelisib; 71% ofatumumab).
Of the patients randomized into each arm, 158 and 155 patients initiated treatment with duvelisib and ofatumumab, respectively (Table 2; supplemental Figure 1). As of the 19 May 2017 data cutoff date, 124 duvelisib-treated patients had discontinued treatment, with the most common reasons being AEs (35%), disease progression (22%), subject withdrawal (8%), and death (8%). All ofatumumab-treated patients had discontinued treatment; most (67%) completed treatment as per protocol, whereas others discontinued because of disease progression (20%), subject withdrawal (5%), and AEs (4%). At the time of disease progression, 8 (5%) duvelisib patients opted to cross over to ofatumumab therapy, and 89 (57%) ofatumumab-treated patients opted to cross over to duvelisib therapy in a separate extension study. Efficacy and safety data for that study will be reported separately.
Efficacy
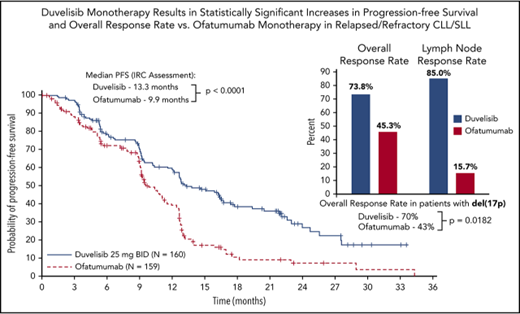
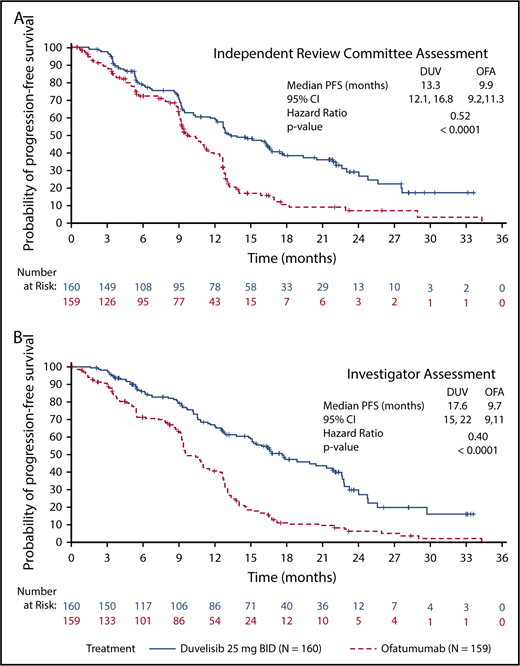
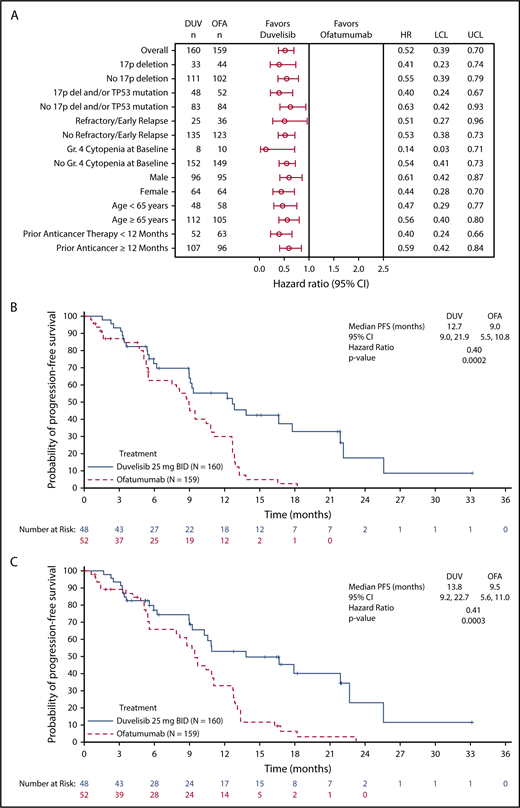
The primary efficacy end point for this study was PFS as determined by IRC, with ORR and OS as key secondary end points. With a median overall follow up of 22.4 months for the duvelisib and ofatumumab arms, median PFS by blinded IRC review was significantly longer for the duvelisib arm compared with the ofatumumab arm (13.3 months vs 9.9 months, HR = 0.52, P < .0001) (Figure 1A). The estimated probability of being progression-free at 6 months and 12 months was 78% and 60%, respectively, in the duvelisib arm, and 72% and 39% in the ofatumumab arm. Improvement in PFS with duvelisib compared with ofatumumab was also observed per investigator assessment (17.6 months vs 9.7 months, HR = 0.40, P < .0001; Figure 1B). PFS was also extended with duvelisib in multiple predefined CLL/SLL subgroups examined, including patients with high-risk cytogenetic markers (Figure 2A). In the subset of patients with del(17p)/TP53 mutations, median PFS by IRC assessment was 12.7 months with duvelisib vs 9.0 months for ofatumumab (HR = 0.40, P = .0002), with an estimated probability of being progression-free at 6 months and 12 months of 73% and 55% with duvelisib, and 63% and 30% with ofatumumab (Figure 2B). By investigator assessment, median PFS in patients with del(17p)/TP53 mutations was 13.8 months with duvelisib and 9.5 months with ofatumumab (HR = 0.41, P = .0003) with an estimated probability of being progression free at 6 months and 12 months of 77% and 66% with duvelisib, and 53% and 33% with ofatumumab (Figure 2C). Duvelisib maintained a favorable odds ratio relative to ofatumumab for all subgroups analyzed, including refractory/early relapse (HR = 0.51), baseline grade 4 cytopenia (HR = 0.14), and prior anticancer therapy within the past 12 months (HR = 0.40).
PFS in the study population. Kaplan-Meier curves of PFS assessments in CLL/SLL patients treated with monotherapy duvelisib or ofatumumab. PFS was significantly longer for duvelisib-treated patients compared with ofatumumab-treated patients by assessments of blinded IRC (13.3 months vs 9.9 months, HR = 0.52, P < .0001) (A) and investigators (17.6 months vs 9.7 months, HR = 0.40, P < .0001) (B). BID, twice daily; DUV, duvelisib; OFA, ofatumumab.
PFS in the study population. Kaplan-Meier curves of PFS assessments in CLL/SLL patients treated with monotherapy duvelisib or ofatumumab. PFS was significantly longer for duvelisib-treated patients compared with ofatumumab-treated patients by assessments of blinded IRC (13.3 months vs 9.9 months, HR = 0.52, P < .0001) (A) and investigators (17.6 months vs 9.7 months, HR = 0.40, P < .0001) (B). BID, twice daily; DUV, duvelisib; OFA, ofatumumab.
PFS in selected study subgroups. (A) Forest plot and hazard ratios for PFS per IRC assessment on duvelisib or ofatumumab monotherapy for predefined subgroups within the total study population. Kaplan-Meier curves of PFS per IRC assessment (B) and investigator assessment (C) in the subgroup of patients with del(17p)/TP53 mutation. In this high-risk subgroup, median PFS was 12.7 months and 9.0 months (HR = 0.40, P = .0002) by IRC assessment and 13.8 months and 9.5 months (HR = 0.41, P = .0003) by investigator assessment for duvelisib and ofatumumab, respectively. “Refractory/early relapse” indicates refractory/early relapse to purine analog-based therapy; “prior anticancer therapy” indicates most recent prior anticancer therapy from randomization. LCL, lower confidence limit; UCL, upper confidence limit.
PFS in selected study subgroups. (A) Forest plot and hazard ratios for PFS per IRC assessment on duvelisib or ofatumumab monotherapy for predefined subgroups within the total study population. Kaplan-Meier curves of PFS per IRC assessment (B) and investigator assessment (C) in the subgroup of patients with del(17p)/TP53 mutation. In this high-risk subgroup, median PFS was 12.7 months and 9.0 months (HR = 0.40, P = .0002) by IRC assessment and 13.8 months and 9.5 months (HR = 0.41, P = .0003) by investigator assessment for duvelisib and ofatumumab, respectively. “Refractory/early relapse” indicates refractory/early relapse to purine analog-based therapy; “prior anticancer therapy” indicates most recent prior anticancer therapy from randomization. LCL, lower confidence limit; UCL, upper confidence limit.
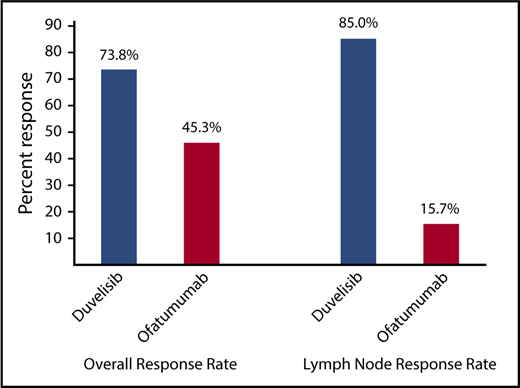
The ORR per IRC response assessment for duvelisib was also significantly higher compared with ofatumumab (73.8% vs 45.3%; P < .0001) (Figure 3). In the duvelisib arm, nearly all responses were PR (72.5%) with the exception of 2 patients: 1 patient achieving a CR (0.6%) and 1 patient achieving a PRwL (0.6%). Responses on the ofatumumab arm were also predominantly PRs (44.7%) with a CR (0.6%) in 1 patient (supplemental Table 2). Duvelisib treatment was particularly effective in targeting the lymph node disease compartment, with a lymph node response by IRC assessment of 85.0% (95% CI, 79.5-90.5) compared with 15.7% (95% CI, 10.1-21.4) in the ofatumumab arm (P < .0001; Figure 3). Median OS was not reached on either treatment arm with a 12-month probability of survival of 86% (HR = 0.99; 95% CI, 0.65-1.50) for both treatments (supplemental Figure 2).
ORR per IRC assessment and lymph node response rate for the total CLL/SLL study population for duvelisib vs ofatumumab monotherapy. Lymph node response was defined as ≥50% decrease in the sum of the products of target lymph nodes. Both the ORR (P < .0001) and the lymph node response rate (P < .0001) were significantly higher in duvelisib-treated patients.
ORR per IRC assessment and lymph node response rate for the total CLL/SLL study population for duvelisib vs ofatumumab monotherapy. Lymph node response was defined as ≥50% decrease in the sum of the products of target lymph nodes. Both the ORR (P < .0001) and the lymph node response rate (P < .0001) were significantly higher in duvelisib-treated patients.
Safety
AEs were assessed during the treatment periods of each arm. Median treatment exposure was approximately twice as long among duvelisib-treated patients (median duration 50 weeks) compared with ofatumumab-treated patients (median duration 23 weeks). Per protocol, ofatumumab treatment did not exceed the 12 doses (∼6 months) specified in the ofatumumab prescribing information. As of the data cutoff, 49% of duvelisib-treated patients had received more than a year of treatment, with 31% and 12% having received 1.5 years and 2 years of treatment, respectively. AEs that occurred in ≥10% patients during these respective AE reporting periods (ie, not exposure matched) are presented in Table 3. Nearly all patients in both arms experienced an AE. The most common hematologic AEs with duvelisib and ofatumumab were neutropenia (33% and 21%), anemia (23% and 10%), and thrombocytopenia (15% and 6%), respectively. The nonhematologic AEs most commonly reported with duvelisib were diarrhea (51%), pyrexia (29%), nausea (23%), and cough (21%). Colitis, as a distinct event from diarrhea, was reported in 13% with 8% of patients having diarrhea directly preceding and/or overlapping with the colitis event. Median time to first event of diarrhea or colitis was ∼4 months and 7 months, respectively. With ofatumumab, infusion-related reaction (19%), cough (14%), and diarrhea, rash, and fatigue (12% each) were the most common nonhematologic AEs.
AEs ≥grade 3 occurred in 87% of duvelisib arm patients and 48% of ofatumumab arm patients. In the duvelisib arm, the most common severe events were neutropenia (30%), diarrhea (15%), pneumonia (14%), and anemia (13%). On the ofatumumab arm, only neutropenia (17%) occurred in ≥10% of patients. As previously observed in the phase 1 study,22,24 severe immune-related toxicities (≥grade 3) were noted in duvelisib-treated patients. In this phase 3 study, the occurrence of severe immune-related toxicities included colitis (12%) and pneumonitis, alanine transaminase, or aspartate transaminase increase (3% each). Although these events were primarily managed with dose interruptions, 60% of patients with pneumonitis or colitis received steroid therapy with resolution reported in nearly all patients at the time of data cutoff. None of these events were fatal.
Infectious AEs were more frequently reported in the duvelisib arm (69% vs 43%) with pneumonia (18%) and URTI (16%) representing the most common events. P jirovecii pneumonia occurred in 3 duvelisib-treated patients and 1 ofatumumab-treated patient, 3 of whom were not receiving prophylaxis at the time of their infections despite being required per protocol and 1 duvelisib-treated patient who discontinued prophylaxis because of intolerance. Of the duvelisib-treated patients who discontinued treatment because of AEs, colitis and diarrhea were the only AEs occurring in ≥5% of patients (both 5%). Treatment discontinuations from the other immune-related toxicities of pneumonitis (2%) and elevated aspartate transaminase levels (1%) were infrequent.
Serious AEs are summarized in supplemental Table 3. Pneumonia was the most frequently reported serious AE in both treatment arms (duvelisib 15%; ofatumumab 3%). There were 19 fatal AEs on the duvelisib arm (supplemental Table 4), 4 of which were assessed by investigators as related to study drug: staphylococcal pneumonia (n = 2) and sepsis and general health deterioration (n = 1 each). On the ofatumumab arm, 7 patients had fatal AEs, although none were attributed to ofatumumab treatment.
Pharmacodynamic measurements
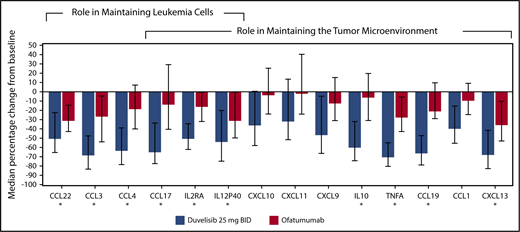
Twenty-four chemokines and cytokines were measured in patient serum samples, and the median percent change from baseline to cycle 2 day 1 was determined to assess treatment-related effects associated with the targeting of leukemia cells and the tumor microenvironment,29,30 as described in the supplemental Methods section. Sixteen chemokines were significantly reduced from baseline in duvelisib-treated patients (P < .05; Figure 4). Ofatumumab-treated patients had levels that were not statistically decreased from baseline, whereas the duvelisib-treated patients had decreased levels that were highly significant: CCL1 (P < .0001), CCL17 (P < .0001), CXCL10 (P < .0002), CXCL11 (P < .0009), CXCL9 (P < .0001), and interleukin-10 (P < .0001). In total, 10 chemokines showed >50% median decreases in duvelisib-treated patients.
Cytokine and chemokine changes in CLL/SLL patients treated with duvelisib or ofatumumab. The median percent change from baseline to cycle 2 day 1 is depicted. The 10 cytokines and chemokines that showed >50% median reductions in duvelisib patients from baseline are denoted with an asterisk.
Cytokine and chemokine changes in CLL/SLL patients treated with duvelisib or ofatumumab. The median percent change from baseline to cycle 2 day 1 is depicted. The 10 cytokines and chemokines that showed >50% median reductions in duvelisib patients from baseline are denoted with an asterisk.
Discussion
In this pivotal phase 3 DUO study, duvelisib monotherapy resulted in statistically significant improvement in PFS and ORR compared with ofatumumab in patients with RR CLL/SLL, including those with del(17p)/TP53 mutations. This improvement was consistently observed across all sensitivity and subgroup analyses for both efficacy end points. In the duvelisib arm, the median PFS as determined using investigator response assessments was notably longer than IRC-assessed PFS (17.6 months vs 13.3 months). This difference likely reflects inherent differences in methodologies used to derive responses and determine progression between the 2 evaluator groups. With the IRC PFS largely based on radiological data, the investigator-derived PFS, incorporating both radiological and real-time clinical assessments, as would be applied in the standard oncology care setting, likely represents a more clinically relevant PFS. Clinically meaningful reductions in target lymph nodes were observed in most patients treated with duvelisib (85%), representing a statistically significant treatment effect over ofatumumab (16%) (P < .0001). Median OS was similar between the 2 treatment arms, which may be explained by the availability of multiple CLL therapies to rescue patients on either arm following disease progression, including administration of duvelisib in a separate, optional extension study to 89 patients who had confirmed progressive disease on ofatumumab in the DUO study.
Based on the study design, the treatment period for duvelisib was longer than ofatumumab (median exposure 50 weeks vs 23 weeks), which resulted in a longer AE reporting period for duvelisib compared with ofatumumab. Of the patients treated with duvelisib, 49%, 31%, and 12% received more than a year, 18 months, and 2 years of treatment, respectively. The duvelisib AE profile observed in this trial was consistent with the drug safety profile to date,22,24 with the majority of AEs presenting as grade 1 or 2 events. Many of the more common AEs, such as cytopenias and constitutional symptoms, are also well-recognized complications of CLL/SLL. Similar to other B-cell receptor pathway inhibitors, infections (particularly pneumonias and URTIs)31,32 were frequently observed on duvelisib, representing the most common severe and serious AEs; 3 patients died because of treatment-related infections. Although a well-recognized major cause of morbidity and mortality in CLL given the humoral immunodepression inherent to the disease33,34 and therapy-induced immunosuppression, infections remain an important risk with duvelisib treatment. The protocol requirement for P jirovecii pneumonia prophylaxis may have mitigated against the risks of these opportunistic infections, which have been reported with other B-cell receptor inhibitors.35-39 Of interest, the 3 duvelisib-treated patients who experienced P jirovecii infections during the study were not receiving prophylaxis because of protocol deviations or medication intolerance.
Several prespecified AEs of interest relating to possible immunomodulatory effects of PI3K-δ/γ inhibition40-45 were closely monitored in this study. Incidences of neutropenia, diarrhea, colitis, transaminase elevations, pneumonitis, and rash with duvelisib therapy were moderate and manageable with early intervention and dose modification as required per protocol. Of these, colitis was the most commonly observed severe (≥grade 3) event, reported in 12% of duvelisib treated patients, and severe cases of pneumonitis, transaminase elevations, and toxic skin eruption/rash were reported in <3%. None of these events were fatal. These potential immune-mediated toxicities, identified in clinical studies with the PI3K-δ specific inhibitor idelalisib, infrequently led to discontinuations of duvelisib therapy.
Malignant B-cell proliferation and survival in CLL/SLL is promoted by PI3K.10-12 The PI3K-δ/γ isoforms are also expressed in cells comprising the tumor microenvironment where they provide supportive signaling.13-16 As a dual PI3K-δ/γ inhibitor, duvelisib has the potential to target cell autonomous mitogenic and survival signaling, as well as the supportive tumor microenvironment that further enables CLL proliferation.30,46 An analysis of patient blood protein profiles showed significant reductions in the levels of serum chemokines and cytokines associated with malignant B cells, consistent with decreases in CLL viability and proliferation, as previously reported.24 Considering the dual inhibitor action of duvelisib, the pattern of chemokine change may reflect the inhibition of both PI3K-δ and PI3K-γ signaling within the tumor microenvironment.
This report of the DUO study data represents the second phase 3 randomized trial to investigate the safety and efficacy of an oral PI3K inhibitor in relapsed CLL patients. In a prior study, idelalisib, an oral inhibitor of the PI3K-δ isoform, in combination with rituximab showed improved PFS compared with rituximab monotherapy (not reached vs 5.5 months) and response rate (81% vs 13%) in patients with relapsed CLL who have clinically significant coexisting medical conditions.47 The incidence of the more common AEs in the idelalisib + rituximab arm, such as cytopenias, constitutional symptoms, and diarrhea, occurred at similar rates in the duvelisib arm of the DUO trial. However, at the time of the idelalisib trial analysis, patients in the idelalisib + rituximab arm had only received study drug for a median of 3.8 months compared with the much longer exposure of duvelisib of nearly a year in the DUO trial. This large difference in drug exposure and observation periods makes it difficult to compare incidences across trials. Additionally, the timing of some immune-mediated toxicities, such as colitis and pneumonitis, which tend to emerge with longer drug exposure, may be more accurately represented in this study with a longer duration of assessment. Interestingly, however, the incidence of high-grade transaminitis AEs with duvelisib was low in this study. The AE profile with duvelisib monotherapy remains consistent across studies22,23 and manageable with appropriate intervention via dose modifications, routine medical care, and prophylactic measures. Additional characterization of key safety events associated with the use of idelalisib40 and PI3K inhibitors as a whole41 has greatly informed the guidance for maximizing safe administration of drugs in this class.
The anti-CD20 monoclonal antibody ofatumumab is approved as monotherapy for the treatment of relapsed CLL and represented an accepted and appropriate standard of care agent for comparing to duvelisib monotherapy at the time of study initiation. However, the use of single-agent ofatumumab for the treatment of relapsed CLL has diminished in recent years, being supplanted by newer agents that target the B-cell receptor pathway. In addition to idelalisib in combination with rituximab, ibrutinib, an oral BTK inhibitor, received full approval in 2014 based on a phase 3 randomized study demonstrating significantly improved PFS compared with ofatumumab (median PFS not reached vs 8.1 months [HR = 0.22]).7 Treatment indications have since expanded to include ibrutinib use as a frontline CLL therapy. Despite advantages of being an effective and well-tolerated therapy, it is not curative, and treatment discontinuations because of disease progression and drug intolerance have been described in published reports.48-50 Given the unfortunate reality that many patients have or acquire resistance or intolerance to currently available, targeted therapies, or have comorbidities that may preclude their safe use, there remains a critical medical need for additional, novel, targeted therapies for patients with RR CLL/SLL, especially those with del(17p)/TP53 mutations. These combined safety and efficacy results suggest that duvelisib monotherapy may offer an effective treatment of CLL/SLL patients in need of additional therapeutic options.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank the patients and families who participated in this study. Veristat Inc. provided biostatistical programming support. Tim Henion of Acumen Medical Communications LLC provided medical writing and editorial assistance, and John Welle provided graphics support.
This work was supported by Verastem Oncology and Infinity Pharmaceuticals Inc.
Authorship
Contribution: I.W.F. provided primary authorship, trial design input, and data interpretation; I.W.F., P.H., M.M., Z.N., A.I., G.E., J.D., B.J.K., C.S.T., Z.G., F.O., S.L., F.B., M.S.D., N.L., U.J., P.G., F.C., C.A.P., A.P.S., A.F.C., and S.S. enrolled patients, performed the research, and contributed to/edited the manuscript; V.M.K. analyzed data and wrote and/or edited the manuscript; B.T. provided statistical outputs; and D.T.W. performed pharmacodynamic analysis and data interpretation.
Conflict-of-interest disclosure: I.W.F. received funds to institution for trial participation from Agios, ArQule, Beigene, Calithera, Celgene, Constellation, Curis, Forma, Forty Seven, Genentech, Gilead, Incyte, Infinity, Janssen, KITE, Merck, Novartis, Pfizer, Pharmacyclics, Portola, Seattle Genetics, Takeda, TG Therapeutics, Trillium, and Verastem. M.M. received personal fees from Janssen and Roche and personal fees and nonfinancial support from AbbVie and Gilead. U.J. received research funding and personal fees from Gilead, Celgene, Roche, and Novartis and personal fees from AbbVie. P.G. received research funding and personal fees from Abbvie, Janssen, and Gilead; research funding from Novartis; and personal fees from Acerta/Astra Zeneca and Beigene. A.F.C. received personal fees from Gilead and was on the advisory board and received personal fees from Verastem. D.T.W. is an employee of Verastem Oncology. V.M.K. was formerly an employee of Infinity Pharmaceuticals and is a consultant for Verastem Oncology. B.T. was a consultant to Verastem Oncology when the analyses were performed. The remaining authors declare no competing financial interests.
Correspondence: Ian W. Flinn, Sarah Cannon Research Institute, 250 25th Ave North, Nashville, TN 37203; e-mail: iflinn@tnonc.com.