Abstract
Rosai-Dorfman-Destombes disease (RDD) is a rare non–Langerhans cell histiocytosis characterized by accumulation of activated histiocytes within affected tissues. RDD, which now belongs to the R group of the 2016 revised histiocytosis classification, is a widely heterogeneous entity with a range of clinical phenotypes occurring in isolation or in association with autoimmune or malignant diseases. Recent studies have found NRAS, KRAS, MAP2K1, and ARAF mutations in lesional tissues, raising the possibility of a clonal origin in some forms of RDD. More than 1000 reports have been published in the English literature; however, there is a lack of consensus regarding approach for the clinical management of RDD. Although in most cases RDD can be observed or treated with local therapies, some patients with refractory or multifocal disease experience morbidity and mortality. Here we provide the first consensus multidisciplinary recommendations for the diagnosis and management of RDD. These recommendations were discussed at the 32nd Histiocyte Society Meeting by an international group of academic clinicians and pathologists with expertise in RDD. We include guidelines for clinical, laboratory, pathologic, and radiographic evaluation of patients with RDD together with treatment recommendations based on clinical experience and review of the literature.
Introduction
Rosai-Dorfman-Destombes disease (RDD) is a rare non–Langerhans cell histiocytosis (LCH) first described in 1965 by a French pathologist, Pierre Paul Louis Lucien Destombes, who reported 4 children and young adults with lymphadenopathy and sinus histiocytosis upon histologic analysis.1 Four years later, Juan Rosai and Ronald Dorfman analyzed 34 cases of the same entity under the name sinus histiocytosis with massive lymphadenopathy.2 Characteristic lesional histiocytes are S100+, CD68+, and CD1a− and demonstrate variable frequency of emperipolesis. Historically, RDD has been considered a self-limited disorder of unknown etiology, although few patients have poor outcomes.3 Patients with classical RDD present with bilateral cervical lymphadenopathy, but 43% of patients with RDD present with extranodal disease.3 RDD is a heterogeneous entity that can occur as an isolated disorder or in association with autoimmune, hereditary, and malignant diseases. Because of the wide clinical spectrum of RDD and the consequent variety of specialists evaluating and treating such patients, there is a need for an evidence-based approach to the evaluation and treatment of this protean condition.
Methods
An English-language search of PubMed was conducted for RDD-related literature from 1965 until the present. Recommendations were derived from a review of the literature and extensive collective clinical experience and deliberation among experts. A group of pathologists, pediatric and adult oncologists, hematologists, and internists, all with extensive (up to 30 years) experience in the treatment of histiocytic disorders, collaborated to establish these recommendations. The 4 pathologists in this group have collectively diagnosed >300 RDD cases. The recommendations proposed here were discussed and approved by the Rare Histiocytoses Steering Committee and Working Group of the Histiocyte Society during its 32nd Annual Meeting in Dublin, Ireland, on 16 October 2016 by members of the North American Consortium for Histiocytosis, a collaborative network of North American physicians studying and treating histiocytic diseases, and by experts from the Euro-Histio-Net. Subsequently, the authors refined these recommendations by an iterative and collaborative process. The absence of large and/or prospective studies in RDD renders the evidence here largely anecdotal, albeit synthesized by experts, and therefore, a grading scheme for consensus recommendations has not been proposed.
Epidemiology
RDD is a rare disease with a prevalence of 1:200 000 and an estimated 100 new cases per year in the United States.4 It is more frequently seen in children and young adults (mean age, 20.6 years), although it has been reported up to age 74 years. RDD is more common in males and in individuals of African descent, with the cutaneous form more common in female Asians.5 Since the publication of the RDD registry (423 cases) by Foucar et al3 in 1990, >1000 reports describing various aspects of this disease have been published.
Pathogenesis
The etiology of RDD is not well defined and is likely not uniform across the spectrum of phenotypes. Historically, clonality studies suggested that lesional RDD cells were polyclonal, reactive, and nonneoplastic.6 Studies have associated RDD with viral infections such as herpes viruses, Epstein-Barr virus, cytomegalovirus, and HIV,7 although a clear link has not been proven. In light of the finding of recurrent BRAF-V600E mutations in Erdheim-Chester disease (ECD), another non-LCH, 23 RDD samples were analyzed and found to be BRAF-V600E wild type.8 Similarly, Chakraborty et al9 did not identify any somatic alterations by whole-exome sequencing of 4 RDD cases. However, recent studies identified NRAS, KRAS, MAP2K1, and ARAF mutations in patients with features of RDD.10-14 Further research is needed to investigate the cell of origin of neoplastic forms of RDD, as has been done in ECD and LCH.15 Figure 1 and Table 1 present the somatic mutations observed to date in RDD. Below, we outline several RDD subtypes and their related phenotypes.
Summary of the diverse kinase mutations documented in RDD. (A) Pie chart illustrating the known activating kinase mutations in RDD (N = 34). (B) Diagrams of somatic mutations described in KRAS and NRAS. (C) Diagram of somatic mutations uncovered in MAP2K1. (D) Diagram of somatic mutation discovered in ARAF.
Summary of the diverse kinase mutations documented in RDD. (A) Pie chart illustrating the known activating kinase mutations in RDD (N = 34). (B) Diagrams of somatic mutations described in KRAS and NRAS. (C) Diagram of somatic mutations uncovered in MAP2K1. (D) Diagram of somatic mutation discovered in ARAF.
Associated diseases
Inherited conditions predisposing to RDD.
Germ line mutations in SLC29A3 have been reported in patients with familial RDD. The SLC29A3 disease spectrum includes familial or Faisalabad histiocytosis, H syndrome, and pigmented hypertrichotic dermatosis with insulin-dependent diabetes, all described as histiocytosis-lymphadenopathy plus syndrome (MIM602782).16 Heterozygous germ line mutations in the FAS gene TNFRSF, which is responsible for autoimmune lymphoproliferative syndrome (ALPS) type I, may be associated with RDD.17,18 These patients have more aggressive manifestations of ALPS, male predominance, and early age at onset, but the RDD-like changes are usually self-limited.17
Neoplasia-associated RDD.
Histologic features of RDD have been observed in patients with Hodgkin and non-Hodgkin lymphomas, where lymphoma and RDD can either precede or follow each other or occur in the same lymph node.19 The disease was also reported after myelodysplastic syndrome20 or bone marrow transplantation for acute leukemia,21 concurrent with cutaneous clear-cell sarcoma22 and concurrent with or following L-group histiocytoses or malignant histiocytoses.23 Small foci of RDD-like histopathology (ie, histiocytes with emperipolesis) are nonspecific; therefore, >10% of a specimen should demonstrate RDD morphology to constitute a neoplasia-associated RDD as a distinct entity rather than a reactive process.
Immune-related RDD.
IgG4-related RDD.
Some forms of extranodal RDD, such as those involving the liver, lungs, or colon, have been associated with an increased number of immunoglobulin G4-positive (IgG4+) plasma cells,26 although other studies have shown a low number of IgG4+ plasma cells and low IgG4/IgG ratios (<40%) as compared with IgG4-related disease samples.27 No clear evidence suggests that the 2 disorders share the same pathogenesis; however, the most recent classification of histiocytoses recommends evaluating the IgG4/IgG ratio in all patients with RDD.28
Pathology
The diagnostic pathologic features of nodal RDD include the sinus expansion of large histiocytes, described by Destombes as possessing ample pale or “watery-clear” cytoplasm with a large hypochromatic nucleus and prominent nucleolus (Figure 2A-C). Nodal RDD is often accompanied by numerous plasma cells in the medullary cords and around the venules, with varying proportions of IgG4/IgG plasma cells. Detailed pathologic review of RDD is provided elsewhere.29 Consistent features, regardless of the site, include the cytomorphology of the large pale histiocytes and their immunophenotype. Emperipolesis, the trafficking of intact leukocytes through the cytoplasm, is a helpful finding but is not required for diagnosis, because it can be focal, especially at extranodal sites, and may be seen focally in other histiocytoses such as ECD,30 juvenile xanthogranuloma, and malignant histiocytoses. Extranodal lesions are usually associated with more fibrosis, fewer RDD histiocytes and less emperipolesis. In such cases, immunostains are needed to highlight the residual RDD histiocytes in a rich lymphoplasmacytic background with stromal fibrosis and a variable xanthomatous histiocytic reaction. These extranodal cases can be difficult to differentiate from ECD and require clinical correlation. The immunophenotype of the large RDD histiocytes is characterized by cytoplasmic and nuclear S100 (Figure 2D) and fascin (Figure 2E) positivity, with CD68 and variable CD163 and CD14 positivity. The cells are CD1a−/CD207− in contrast to LCH. An important aspect of making the diagnosis of nodal RDD is evaluation for superimposed pathology, either within the node itself or other associated conditions.29 The presence of RDD histology is required, but not sufficient, for the diagnosis of RDD, which depends on the appropriate clinical and radiologic context and exclusion of primary malignant disorders in relation to which RDD histology may represent a minor (<10%) reactive process. Because the pathologic features of RDD are variable, compounded by its heterogeneous clinical manifestations, in many instances the primary hurdle to establishing an RDD diagnosis is suspecting the disorder in the first place, particularly for extranodal disease. The presence of slowly progressive or subacutely clinical symptomatology; biopsy specimens demonstrating ostensibly nonspecific inflammation, including lymphoid aggregates and plasma cells with a large histiocytic presence; and clinical findings compatible with those described in “Clinical features” should raise concern for an RDD diagnosis and, in difficult cases, prompt clinical and histopathologic evaluation by experts familiar with the disease.
Pathologic and clinical features of RDD. (A-E) Representative images of nodal RDD from tissue biopsies (A-B) and fine-needle aspiration (C-E). (A) Mixed RDD/LCH case with sinus expansion. The large RDD histiocytes display conspicuous emperipolesis with pale cytoplasm, as compared with the intermixed LCH cells with dense eosinophilic cytoplasm and convoluted nuclei (original magnification [OM] ×400; hematoxylin and eosin [H&E] stain). (B) The RDD histiocytes show pale watery-clear cytoplasm, a central round nucleus with a conspicuous nucleolus, and emperipolesis (OM ×1000; H&E stain). Cell block preparation shows clusters of RDD histiocytes (OM ×400; H&E stain) (C), with nuclear and cytoplasmic staining for S100 (OM ×1000) (D) and fascin (OM ×1000) (E); the trafficking intact leukocytes are negative. (F) A child with immunodeficiency and RDD with massive cervical lymphadenopathy. (G) RDD of the skin showing red nodular lesions. (H) Tongue enlargement resulting from oral RDD.
Pathologic and clinical features of RDD. (A-E) Representative images of nodal RDD from tissue biopsies (A-B) and fine-needle aspiration (C-E). (A) Mixed RDD/LCH case with sinus expansion. The large RDD histiocytes display conspicuous emperipolesis with pale cytoplasm, as compared with the intermixed LCH cells with dense eosinophilic cytoplasm and convoluted nuclei (original magnification [OM] ×400; hematoxylin and eosin [H&E] stain). (B) The RDD histiocytes show pale watery-clear cytoplasm, a central round nucleus with a conspicuous nucleolus, and emperipolesis (OM ×1000; H&E stain). Cell block preparation shows clusters of RDD histiocytes (OM ×400; H&E stain) (C), with nuclear and cytoplasmic staining for S100 (OM ×1000) (D) and fascin (OM ×1000) (E); the trafficking intact leukocytes are negative. (F) A child with immunodeficiency and RDD with massive cervical lymphadenopathy. (G) RDD of the skin showing red nodular lesions. (H) Tongue enlargement resulting from oral RDD.
Clinical features
Classic (nodal) RDD.
Most patients with RDD present with bilateral, massive, and painless cervical lymphadenopathy (Figure 2F) with or without intermittent fevers, night sweats, and weight loss.31 Mediastinal, axillary, and inguinal nodes may also be involved, but retroperitoneal lymphadenopathy is uncommon.32 Prognosis has been found to correlate with the number of nodal groups involved by RDD.3
Extranodal RDD.
Extranodal involvement has been reported in 43% of RDD cases.3 A brief discussion of extranodal sites affected by RDD follows, with differential diagnoses for each site. Other histiocytoses such as LCH and ECD should be considered as alternative or associated diagnoses for all sites. Multisystem involvement occurs in 19% of cases, and prognosis is correlated with the number of extranodal systems involved.3
Cutaneous manifestations.
The skin is involved in 10% of extranodal RDD cases, and isolated cutaneous disease is rare.31 Lesions are typically slow growing, painless, nonpruritic nodules, plaques, or papules with coloration varying from yellow to red to brown (Figure 2G). Any skin site can be affected. The differential diagnosis includes acne vulgaris, varicella-zoster virus, sarcoidosis, cutaneous lymphoma, and metastasis.31
Intracranial, spinal, CNS, and ophthalmic manifestations
Central nervous system (CNS) involvement occurs in <5% of cases, with 75% occurring as intracranial and 25% as spinal lesions. Neurologic RDD has been reported in >300 cases33 and usually occurs in older patients and without lymphadenopathy.34 Symptoms include headaches, seizures, gait difficulty, motor or sensory abnormalities, and cranial nerve deficits, usually evolving over weeks or months.33 Familial cases are associated with damage to the auditory nerve pathway and deafness.16,35 The most common radiographic appearance of intracranial RDD is a solitary extraaxial, homogeneously enhancing dural mass (Figure 3A) mimicking a meningioma,36,37 although RDD can cause diffuse pachymeningitis. Parenchymal lesions are frequently infratentorial (brainstem and pons; Figure 3B),38 whereas supratentorial, intraventricular, and multifocal lesions are rare.39,40 Cerebrospinal fluid is often unremarkable although may show lymphocytic pleocytosis, elevated protein, low glucose, and emperipolesis.37
Radiographic features of RDD. (A) Gadolinium-enhanced coronal T1-weighted magnetic resonance imaging (MRI) demonstrates a dural-based lesion at the base of the right frontal lobe (blue arrow). (B) Patchy enhancing lesions in the brainstem on gadolinium-enhanced axial T1-weighted MRI (green arrow). (C) Gadolinium-enhanced axial T1-weighted MRI demonstrates lesions in the bilateral cavernous sinuses and Meckel’s cave (red asterisks) as well as the left orbit (red arrow), with resulting proptosis. (D) Axial fused [18F]fluorodeoxyglucose (FDG)–positron emission tomography (PET)/computed tomography (CT) demonstrates a hypermetabolic dural-based paraspinal mass with involvement of the osseous elements of the thoracic spine with foraminal extension. (E) Response to 12 weeks of 1 mg/kg per day of prednisone is demonstrated, with near resolution of hypermetabolism and regression of the tumor. (F) Partly T2-weighted bright-blood image shows an oval mass (black arrow) in the left atrium (LA) arising from the central part of the heart at the atrioventricular junction. LV, left ventricle; RA, right atrium; RV, right ventricle.
Radiographic features of RDD. (A) Gadolinium-enhanced coronal T1-weighted magnetic resonance imaging (MRI) demonstrates a dural-based lesion at the base of the right frontal lobe (blue arrow). (B) Patchy enhancing lesions in the brainstem on gadolinium-enhanced axial T1-weighted MRI (green arrow). (C) Gadolinium-enhanced axial T1-weighted MRI demonstrates lesions in the bilateral cavernous sinuses and Meckel’s cave (red asterisks) as well as the left orbit (red arrow), with resulting proptosis. (D) Axial fused [18F]fluorodeoxyglucose (FDG)–positron emission tomography (PET)/computed tomography (CT) demonstrates a hypermetabolic dural-based paraspinal mass with involvement of the osseous elements of the thoracic spine with foraminal extension. (E) Response to 12 weeks of 1 mg/kg per day of prednisone is demonstrated, with near resolution of hypermetabolism and regression of the tumor. (F) Partly T2-weighted bright-blood image shows an oval mass (black arrow) in the left atrium (LA) arising from the central part of the heart at the atrioventricular junction. LV, left ventricle; RA, right atrium; RV, right ventricle.
Spinal dural or epidural lesions are most common in the cervical and thoracic regions41 (Figure 3C-D) and present with myelopathy or symptoms of spinal cord compression. Although CNS RDD can have a rapidly progressive and even fatal course, many patients will have a favorable outcome after surgical resection when this is feasible.34
Head and neck manifestations.
Involvement of the nasal cavity and paranasal sinuses occurs in 11% of RDD cases3 and is more common in patients of Asian descent. Symptoms of sinonasal RDD include nasal obstruction, epistaxis, nasal dorsum deformity, facial asymmetry, and aural fullness.43 Oral cavity involvement can present as soft and hard palate nodules, gingival and oral mucosa swelling, tongue enlargement (Figure 2H), thickened mucosa of the oropharynx, enlarged tonsils, or frequent tonsillitis.3 Other less frequently involved sites include the salivary and parotid glands, larynx, pharynx, thymus, and thyroid gland, which can cause symptoms related to mass effect.44,45
Intrathoracic manifestations.
Intrathoracic RDD is described in 2% of patients, usually with concurrent lymphadenopathy.46 Manifestations include interstitial lung disease, pulmonary nodules, tracheobronchial disease, and pleural effusions with an obstructive pattern on pulmonary function tests.47 Symptoms include chronic dry cough, progressive dyspnea, or acute respiratory failure. Pulmonary RDD can mimic primary lung cancers, interstitial lung disease or organizing pneumonias, sarcoidosis, granulomatous polyangiitis, rheumatoid arthritis–related lung disease, and mycobacterial and fungal infections.46 RDD affecting the lower respiratory tract can have an aggressive phenotype, with a mortality rate of almost 45%.3 Cardiac involvement with RDD is rare, occurring in 0.1% to 0.2% of cases (Figure 3F).48
Retroperitoneal and genitourinary manifestations.
The kidneys are affected in 4% of RDD cases, with a discrete mass or diffuse infiltration.49,50 Symptoms include hematuria, flank pain, abdominal fullness, renal failure, hypercalcemia, or nephrotic syndrome caused by amyloidosis or renal vein thrombosis.51-54 Hydronephrosis and ureteral obstruction can occur.55 The differential diagnosis of renal RDD includes ECD, lymphoma, renal cell carcinoma, tuberculosis, IgG4-related disease, or metastatic tumor. Patients with renal involvement have a poor prognosis, with 40% mortality rate.3
GI manifestations.
Gastrointestinal (GI) involvement occurs in <1% of RDD cases, most commonly in middle-age women with concurrent lymphadenopathy or other extranodal disease.3 GI RDD can be solitary or segmental and has a predilection for the ileocecal area, appendix, and distal colon, with most cases being located beyond the pylorus.58,59 Symptoms include hematochezia, constipation, abdominal pain, abdominal mass, and intestinal occlusion, although asymptomatic cases have been identified after colonoscopy or appendectomy.59,60 Almost 20% of the reported patients in 1 series died as a result of disease.58 Pancreatic or hepatic involvement is reported but extremely rare.3,61,62
Bone manifestations.
Bone involvement occurs in 5% to 10% of RDD cases, typically in association with nodal disease.3,63 Bone pain is common, whereas pathologic fractures are rare.51 Bone lesions typically occur in the metaphysis or diaphysis, are osteolytic or mixed lytic/sclerotic, and have a narrow zone of transition. Soft tissue extension can occur. The clinical differential diagnosis includes chronic osteomyelitis, fibrous dysplasia, lymphoma, and Ewing sarcoma. Lesions in the femurs and tibia should raise concern for ECD. The prognosis of bony RDD is generally good.64
Hematologic manifestations.
Baseline evaluations
Imaging and laboratory studies.
The diagnostic and staging evaluation of patients with newly diagnosed RDD (Table 2) should include an assessment of disease extent, as well as evaluation for conditions either known to be associated with RDD, particularly autoimmune disorders, or known to contain an RDD-like reactive component secondary to malignancies. A comprehensive medical history and physical and neurologic examinations should be performed. In children, a chest X-ray with neck and abdominal ultrasounds are routinely performed initially. For older patients, CT of the neck/chest/abdomen and pelvis is recommended. RDD lesions are known to be FDG-avid, including extranodal areas,66 and FDG-PET/CT is used by some investigators for initial staging when possible, similarly to patients with ECD.30 Of note, RDD lesions can have appearance and avidity on FDG-PET similar to those of intermediate- and high-grade lymphomas, and these should be diagnostic considerations. Rather than skull-to-thigh scanning, full-body PET including the distal extremities should be performed for comprehensive osseous evaluation. However, there is no consensus among the authors about the benefits of PET/CT in patients with RDD as compared with anatomic imaging. In children, efforts must be made to minimize radiation exposure and need for anesthesia. Whole-body MRI is usually recommended instead of CT scans, and PET scans should be used judiciously. Patients with orbital or neurologic symptoms should have a gadolinium-enhanced MRI of the brain, orbits, or total spine depending on the localizing symptoms; screening MRI of the brain and spine with contrast may be appropriate to identify asymptomatic neurologic involvement. Dedicated organ-specific imaging (ie, MRI of the heart or abdomen) may be necessary to evaluate structural lesions identified but poorly characterized by CT or PET.
Laboratory evaluation should include comprehensive metabolic panel, a complete blood count with differential, erythrocyte sedimentation rate, C-reactive protein, and quantitative immunoglobulin levels. Serologies for HIV and hepatitis B and C are suggested to exclude these as associated diagnoses. Testing for antinuclear antibodies and rheumatoid factor is suggested, because screening for associated systemic lupus erythematous or idiopathic juvenile arthritis and further evaluation for evidence of autoimmunity should be carried out if other diagnoses are considered on the basis of medical history and physical examination. Bone marrow aspirate and biopsy are required only for patients with unexplained cytopenias or abnormal peripheral blood cells.
Acquisition and analysis of lesional tissue.
It is important that sufficient tissue be acquired to establish an RDD diagnosis and that biopsies be reviewed by a pathologist familiar with RDD. Flow cytometry and cytogenetic testing may be required to rule out a lymphoproliferative disorder. Immunohistochemistry for IgG4 should be performed when pathology samples show an enriched plasma-cell presence. In the event of severe or refractory disease, lesional tissue should be analyzed to detect gain-of-function mutations of genes of the MAPK pathway amenable to targeted therapies (including at least KRAS, NRAS, HRAS, ARAF, BRAF, and MAP2K1).
Treatment
General principles.
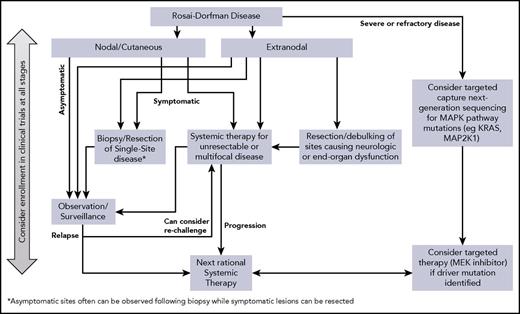
No uniform approach has been delineated for RDD, and treatment is best tailored to the individual clinical circumstances. Accordingly, notions of first-line and second-line treatments are not felt to be applicable to RDD. Below we summarize therapeutic strategies and their implementation (Table 3) and propose a management algorithm (Figure 4).
Observation.
After the diagnosis of RDD is established, observation is reasonable in many cases, because 20% to 50% of patients with nodal/cutaneous disease will have spontaneous remissions.67,68 This strategy is suitable for patients with uncomplicated lymphadenopathy or asymptomatic cutaneous RDD and potentially for those with asymptomatic disease in other sites.
Surgery.
Surgery for RDD is usually limited to biopsy, but resection can be curative for unifocal disease, and debulking may be warranted for upper airway obstruction, spinal cord compression, or large lesions causing end-organ compromise. Long-term remissions with resection alone have been reported in isolated intracranial disease.69 The most effective treatment of cutaneous RDD is surgical excision.31 Endoscopic resection of sinonasal RDD can achieve symptomatic control and restoration of function.43 In cases of multifocal disease, surgical resection of single foci should be reserved for bulky disease with neurologic or end-organ dysfunction.
Corticosteroids.
Steroids are usually helpful in reducing nodal size and symptoms, although responses have been variable. The optimal corticosteroid (prednisone or dexamethasone) dose and duration are not clearly defined. Prednisone (40-70 mg per day) has produced complete or partial responses in cases of orbital, CNS, bone, and autoimmune hemolytic anemia–associated disease.70,71 Compared with other immune diseases (eg, sarcoidosis), therapeutic prednisone doses are usually higher (>0.5 mg/kg per day). Similarly, dexamethasone (8-20 mg per day) was effective in cases of CNS RDD and hilar lymphadenopathy.72,73 One viable approach is to treat to the best observed response, followed by slow taper. An anecdotal response to intralesional steroids has been reported in an adult with orbital RDD and optic nerve compression.74 Nevertheless, other reports of orbital, tracheal, renal, or soft tissue RDD have shown a failure to respond to steroids.75,76 Furthermore, relapses of RDD lesions can sometimes occur after a short period of interruption. Anecdotally, the experience of the authors is that patients with extranodal disease do not generally demonstrate a durable response to steroids alone.
Sirolimus.
Mammalian target of rapamycin is a critical pathway for the control of proliferation and cytokine production from immune cells and has been found to be dysregulated in RDD.77 Sirolimus and prednisone induced objective responses and disease stabilization in 80% of patients with ECD in 1 report,78 and sirolimus was found to be beneficial in a child with resistant RDD and recurrent autoimmune cytopenias.77 The use of this agent for ALPS- or autoimmune-associated RDD requires further study.
Chemotherapy.
Treatment of RDD with chemotherapeutic agents has shown mixed results. Although chemotherapy is generally reserved for refractory or relapsed cases, sometimes it is used as initial therapy in disseminated or life-threatening disease. Anthracyclines and alkylating agents have little efficacy, whereas vinca alkaloids have shown variable responses.67 Low-dose MTX and 6-MP administered in combination were effective in few patients.67,79 Sustainable remissions after regimens containing vinblastine/MTX/6-MP and 6-thioguanine,80 vinblastine/prednisone/MTX/6-MP,81 or vinorelbine/MTX82 have been reported. Single-agent 6-MP was effective in halting disease in an adult with orbital and intracranial RDD.83 Furthermore, long-term remission of intracranial RDD has been reported after postsurgical maintenance with CHOP (cyclophosphamide, doxorubicin, vincristine, and prednisone)–like regimens.84 A patient with multiply-relapsed nodal RDD responded to cytarabine/prednisone/vincristine followed by MTX/6-MP maintenance.85 Successful treatment of refractory cutaneous RDD was reported with single-agent vincristine86 and low-dose MTX.87 In addition, azathioprine and interferon-α induced long-term remissions in patients with RDD.88,89 However, interferon-α in combination with chemotherapy failed to induce any response in another report.67 Therefore, these agents could be considered for steroid-refractory disease or early recurrence after interruption of steroids or when steroids are contraindicated.
Nucleoside analogs cladribine and clofarabine have induced responses in RDD.51,90-93 They impair the function of monocytes through inhibition of interleukin-6 (IL-6), IL-1β, and tumor necrosis factor α (TNF-α) production. Cladribine (2.1-5 mg/m2 per day for 5 days every 28 days for 6 months) induced prolonged remissions in cases of recurrent or refractory systemic RDD,51,90-92 and clofarabine (25 mg/m2 per day for 5 days every 28 days for 6 months) was effective as salvage therapy in a series of patients with refractory or relapsed RDD.93 These agents should be considered in severe or refractory cases when the potential benefit justifies their myelosuppressive toxicity. A multicenter prospective study (registered at www.clinicaltrials.gov as #NCT02425904) is being conducted by the North American Consortium for Histiocytosis to study the efficacy and safety of clofarabine salvage in patients with histiocytoses, including RDD.
Immunomodulatory therapy.
TNF-α inhibitors thalidomide and lenalidomide have shown promising results in RDD because the identification of high levels of TNF-α and IL-6 provides a rational basis for their effectiveness. A recent review showed that low-dose thalidomide (100 mg per day) was effective in refractory cutaneous RDD.94 However, responses to thalidomide have not been universal, and optimal dosing and duration of this drug remain unknown. Lenalidomide recently showed an excellent response in an adult with multiply-refractory nodal and bone RDD and may be more tolerable than thalidomide (fewer skin rashes and less neuropathy), although more myelosuppressive.95
Targeted therapies.
Imatinib mesylate, a tyrosine kinase inhibitor, showed some activity in 1 patient with refractory RDD. Lesional histiocytes were positive for the imatinib target proteins PDGFRB and KIT by immunohistochemistry, but no concurrent mutation was found.98 In another case, cutaneous RDD was refractory to imatinib.99
Unlike ECD and LCH, BRAF-V600E mutations have not been observed in RDD8-14 ; thus, the use of BRAF inhibitors is not relevant. MEK inhibition has shown preliminary activity in BRAF–wild-type ECD100 and in an adult with KRAS-mutated RDD.101 A phase 2 trial of cobimetinib for patients with BRAF–wild-type histiocytoses, including RDD, is ongoing (registered at www.clinicaltrials.gov as #NCT02649972), with promising early results.102 The robust activity of targeted therapies in other histiocytoses raises interest in their potential for RDD, especially in cases with demonstrated somatic mutations; however, currently, the broad applicability of tumor sequencing and targeted treatments has not yet been established.
Radiotherapy.
Radiotherapy has modest efficacy in RDD, although it can be beneficial in refractory soft tissue and orbital bone disease with visual compromise97 or resistant airway obstruction or to palliate local symptoms.67,103 Radiotherapy has also been implemented in patients whose symptoms persist or recur after resection of isolated disease and in those who are not suitable candidates for surgery and/or when other treatments are contraindicated.33,64 No standard doses of radiotherapy have been established, but doses between 30 and 50 Gy have been employed.103
Treatment course, disease surveillance, and prognosis
The optimal duration of steroids or other systemic therapies for RDD is not known. Six to 12 months of systemic therapy followed by observation, assuming tolerance and a favorable response to treatment, is a reasonable approach. When initiating treatment, a first response assessment should be carried out within 4 months; if disease stabilizes or is in remission, interval of surveillance can be extended to 6 to 12 months.
Data are insufficient to characterize the prognosis of RDD in great detail. Outcomes are usually favorable, particularly for cases of nodal and cutaneous disease, which are often self-limited. Other patients experience an unpredictable clinical course, with alternating periods of remission and reactivation that may last years. In the largest series, conducted by Foucar et al3 in 1990, 17 (7%) of 238 patients died as a result of direct complications of their disease, infections, or amyloidosis.3 In a review by Pulsoni et al67 in 2002, 10 (12%) of 80 patients died as a result of RDD.67 Patients with multifocal and extranodal RDD, particularly those with kidney, liver, or lower respiratory tract disease, seem to have an unfavorable prognosis. Thus, intensive systemic chemotherapy, targeted therapies, and investigational agents may be justified in this context. Further elucidation of the role of these therapies in refractory RDD may mitigate the poor prognosis of some of these cases.
Conclusions
RDD is a rare and heterogeneous disorder presenting many diagnostic and therapeutic challenges. The recent biologic and molecular advances in other histiocytoses such as LCH and ECD have not been matched in RDD; therefore, there is urgent need to continue investigating the mutational landscape of RDD and its therapeutic relevance. Multidisciplinary collaboration is often vital to the evaluation and management of patients with RDD, and systematic investigation of novel therapies for RDD is needed. Comprehensive RDD evaluation involves careful medical history, physical examination, imaging studies, and laboratory evaluations to determine the extent of disease and presence of cooccurring disorders. Many cases of RDD can be managed with observation alone, whereas other patients require a variety of immunomodulatory and antineoplastic agents. The roles of tumor sequencing and targeted therapies, such as MEK inhibitors, are promising and require further study. The ongoing International Registry for the Rare Histiocytic Disorders (registered at www.clinicaltrials.gov as #NCT02285582) aims to better understand the clinicopathologic features, treatment strategies, and outcomes of patients with RDD and other rare histiocytic disorders.
Acknowledgments
This work was supported by a National Institutes of Health, National Cancer Institute Core Grant (P30 CA008748) awarded to Memorial Sloan Kettering Cancer Center. B.H.D. is supported by the American Society of Hematology Senior Research Training Award for Fellows and the New York State Council on Graduate Medical Education Empire Clinical Research Investigator Program Fellowship from Memorial Sloan Kettering Cancer Center. E.L.D. is supported by the Frame Fund generously donated to Memorial Sloan Kettering Cancer Center. J.A.W. is supported in part by the Women’s Auxiliary Millennium Chair in Haematology/Oncology at The Hospital for Sick Children.
Authorship
Contribution: The paper was written by O.A., E.L.D., E.J., K.L.M., J.H., and J.P.; all authors participated in editing the manuscript and providing expert recommendations; histopathologic images were provided by J.P.; genome sequencing image and table were provided by B.H.D.; clinical images were provided by O.A. and J.H.; and radiographic images were provided by E.L.D. and O.A.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Oussama Abla, Division of Hematology/Oncology, The Hospital for Sick Children, University of Toronto, 555 University Ave, Toronto, ON M5G1X8, Canada; e-mail: oussama.abla@sickkids.ca.
References
Author notes
K.L.M., J.H., and E.L.D. contributed equally to the paper.


![Figure 2. Pathologic and clinical features of RDD. (A-E) Representative images of nodal RDD from tissue biopsies (A-B) and fine-needle aspiration (C-E). (A) Mixed RDD/LCH case with sinus expansion. The large RDD histiocytes display conspicuous emperipolesis with pale cytoplasm, as compared with the intermixed LCH cells with dense eosinophilic cytoplasm and convoluted nuclei (original magnification [OM] ×400; hematoxylin and eosin [H&E] stain). (B) The RDD histiocytes show pale watery-clear cytoplasm, a central round nucleus with a conspicuous nucleolus, and emperipolesis (OM ×1000; H&E stain). Cell block preparation shows clusters of RDD histiocytes (OM ×400; H&E stain) (C), with nuclear and cytoplasmic staining for S100 (OM ×1000) (D) and fascin (OM ×1000) (E); the trafficking intact leukocytes are negative. (F) A child with immunodeficiency and RDD with massive cervical lymphadenopathy. (G) RDD of the skin showing red nodular lesions. (H) Tongue enlargement resulting from oral RDD.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/131/26/10.1182_blood-2018-03-839753/4/m_blood839753f2.jpeg?Expires=1769186669&Signature=i0SE2dWXnN4Tq0SMpgRFOxgcrVLZPfDFkMc-mSlUTasIJhRolXB6hi6uZtbtKwuakqNUfWdzK-srlrKuOX0FCssd92jgw-~xbWrvzT9Tx4eaBZX~uIJaCW1nc6v2tl64YS8~nqUqBtwcNz-rU7GDhu6MyTE0bj0AfJ3MSWGOYPtvpj4o7xLaIaoIRttPqyKifmP6HnW3O7dXv1Xpj9jjUVcABCffuyk2Qwg~HIDUCx0BozqrQfMXDHkG-fAI2vQwTH8AxYEEiKMYEfh-Q2LW7QPjeCyA6049CDzeu-7m3umUbUwfaroWjsVIwxH9yTrwZRekWTxJ1mZ5m415cnVZRw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 3. Radiographic features of RDD. (A) Gadolinium-enhanced coronal T1-weighted magnetic resonance imaging (MRI) demonstrates a dural-based lesion at the base of the right frontal lobe (blue arrow). (B) Patchy enhancing lesions in the brainstem on gadolinium-enhanced axial T1-weighted MRI (green arrow). (C) Gadolinium-enhanced axial T1-weighted MRI demonstrates lesions in the bilateral cavernous sinuses and Meckel’s cave (red asterisks) as well as the left orbit (red arrow), with resulting proptosis. (D) Axial fused [18F]fluorodeoxyglucose (FDG)–positron emission tomography (PET)/computed tomography (CT) demonstrates a hypermetabolic dural-based paraspinal mass with involvement of the osseous elements of the thoracic spine with foraminal extension. (E) Response to 12 weeks of 1 mg/kg per day of prednisone is demonstrated, with near resolution of hypermetabolism and regression of the tumor. (F) Partly T2-weighted bright-blood image shows an oval mass (black arrow) in the left atrium (LA) arising from the central part of the heart at the atrioventricular junction. LV, left ventricle; RA, right atrium; RV, right ventricle.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/131/26/10.1182_blood-2018-03-839753/4/m_blood839753f3.jpeg?Expires=1769186669&Signature=GZfNosICwDCcvn5WznjdVxfU0qtDb2iRtOOWjdl4t7Qxtm7B-X9XDu1rKHODEBusIKKRPhtt3Ylvy4cEcQZE-IrOsOEUPJTUdvJGnPqZ9~p6-qB89Auurb22CxnU92X6tKb86tIJEl9ZeD7c6svXcgB8mCaNQ3fYkvH8V5kYLe5KdGKiyFusjgQEG6eMru~seD1Uu5FQV3uly-AmXMbmlcTXypbqp2nUZ624FFWsCBgtQPubF8jxasIy9-cCgO-5u3iLHyPHH6Srq~HCXWpqYY3DQSaX6jmdOXv9ydQ15J6c~1vqh1TbUXe8-aO3FQ~odz0etX~OPQ71SHjVtkJVnA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)