Key Points
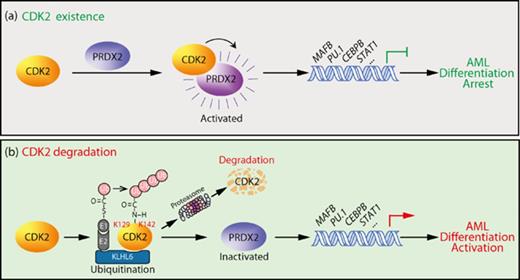
CDK2 is ubiquitinated by KLHL6 and undergoes ubiquitin-dependent proteasome degradation in the therapeutic differentiation process of AML.
CDK2 arrests myeloid cell differentiation via activating PRDX2, and CDK2 inhibition drives differentiation in 5 major subtypes of AML.
Abstract
A distinct hallmark of acute myeloid leukemia (AML) is the arrest of leukemic myeloblasts at an immature stage of development. Therapies that overcome differentiation arrest have emerged as a powerful strategy for treating AML, but targeting leukemia differentiation remains challenging, mainly because of an incomplete mechanistic understanding of the process. Here, we unveil a new role for cyclin-dependent kinase 2 (CDK2) in blocking myeloid differentiation in AML. We show that among several interphase CDK, only CDK2 undergoes ubiquitin-dependent proteasome degradation, which is accompanied by AML cell differentiation. By using the yeast 2-hybrid system and functional analyses, KLHL6 was identified as a specific E3 ubiquitin ligase regulating the degradation of CDK2. Importantly, inhibiting CDK2, but not other cyclin-dependent kinases CDK1/4/6, effectively induced granulocytic differentiation in AML cell lines and 5 major subtypes of primary patient-derived AML samples. Mechanistically, CDK2 depletion led to the reactivation of differentiation pathway translation, and the differentiation blockade function of CDK2 may be achieved directly by maintaining the activity of PRDX2. Finally, CDK2 depletion arrested tumor growth of AML cells in nude mice and extended survival in both AML cell line and PDX-AML cells derived xenograft mouse models. Thus, our work not only provides experimental evidence for validating CDK2 as a potential therapeutic target for differentiation, but also uncovers the biological function of the CDK2-PRDX2 axis in blocking AML differentiation.
Introduction
Acute myeloid leukemia (AML) is a clinically devastating and mostly incurable disease currently; it accounts for ∼80% of all adult acute leukemias. Despite advances in diagnosis and therapeutics, the 5-year survival rate of an adult with AML is only 30%. The development of new and effective anti-AML therapies is clearly required.
One of the hallmarks of AML is a lack of cellular differentiation, suggesting that preventing differentiation arrest might be potential therapy in AML. In the small subset (10%) of patients with acute promyelocytic leukemia (APL), a cytogenetically distinct subtype of AML characterized by t(15; 17)-associated PML-RARA fusion, has been successfully treated with all-trans-retinoic acid (ATRA) to differentiate leukemic blasts. The dramatic success and clinical impact of this differentiation therapy inverted the survival curve for APL patients; where APL was once among the worst prognostic subsets of AML, it now has the best prognosis with overall survival rates in excess of 85%.1,2 An unmet challenge is to identify similar differentiation therapy strategies for the remaining 90% of AML patients.
Cell proliferation and differentiation are inversely correlated processes during normal development. All leukemia cells exhibit both uncontrolled proliferation and a lack of terminal differentiation. Because exit from the cell cycle is a necessary step in terminal differentiation, the downregulation of a mitogenic factor may be expected in this process. cyclin-dependent kinase 2 (CDK2), a member of the cyclin-dependent kinases, is activated by the formation of a complex with a cyclin and is required for G1 phase progression and entry into S phase. Several independent studies of multiple cell types have indicated a novel role for CDK2 in differentiation, regardless of its role in the cell cycle. For example, CDK2 loss is shown to accelerate the development of the adult mouse central nervous system.3,4 The double knockout of CDK2 and CDK4 induces neurogenic divisions in neural stem cells.5 In addition to its role in the nervous system, CDK2 activity might be crucial for cell fate decisions in embryonic stem cells.6,7 These observations strongly support the significance of CDK2 reduction in the regulation of normal cell differentiation. However, it is unknown whether such a differentiation blocking function of CDK2 also exists in cancer cells, especially in leukemia cells.
In fact, the central role attributed to CDK2 in cell-cycle progression has been challenged by the observation that mice lacking this kinase develop normally.8,9 The compensation for interphase CDK (CDK2, CDK4, and CDK6) function by CDK1 may explain why cells lacking CDK2 can proliferate in culture.10 Therefore, it is reasonable to predict that CDK2 may have novel biological functions other than cell-cycle regulation. In this study, we investigated the role of CDK2 in the cellular differentiation of AML cells. We show that CDK2 was specifically degraded upon the therapeutic differentiation of AML cells. The depletion of CDK2 overcame the myeloid differentiation blockade of AML cells. Herein, our work offers new insights into the role of CDK2 in AML differentiation and suggests that targeting CDK2 may be a novel therapeutic approach targeting differentiation in AML.
Materials and methods
Human patient blasts, cells, and cell culture
Primary blasts (Leu-1-19) from the bone marrow of patients (Children's Hospital of Zhejiang University and the First Affiliated Hospital of Zhejiang University) were isolated using lymphocyte monocyte separation medium (Awardbio, Shanghai, China). Details for cells and cell culture are available in the supplemental Methods (available on the Blood Web site).
Plasmids and reagents
See the supplemental Methods for plasmids and reagents.
Western blotting, immunoprecipitation, and real-time polymerase chain reaction
Experiments were conducted as described previously11 ; details are available in the supplemental Methods.
Cellular proliferation and differentiation analyses
The total cell number and viability were determined by trypan blue exclusion with manual counting in Burker chambers. Cellular differentiation was determined by assessing CD11b expression, nitro blue tetrazolium (NBT) reduction assays, morphological changes, and bacterial killing ability (supplemental Methods).
In vitro ubiquitination and degradation assays
In vitro ubiquitination assays were conducted as previously described with minor modifications.12 See the supplemental Methods for details.
Lentivirus production and transduction
Virion production, titration, and transduction were performed as described previously13 ; details are available in the supplemental Methods.
Microarray analysis
Microarray analysis was performed as described previously14 ; NB4 cells were subjected to GeneChip PrimerView Human Gene Expression Array (Affymetrix, Santa Clara, CA), as detailed in the supplemental Methods. The raw data were deposited in Gene Expression Omnibus under the accession number GSE64077 (http://www.ncbi.nlm.nih.gov/geo).
Yeast 2-hybrid screen
To screen for E3 ubiquitin ligases that might ubiquitinate CDK2, yeast 2-hybrid (Y2H) screening was performed as previously described15 ; details are available in the supplemental Methods.
Recombinant PRDX activity assay
PRDX peroxidase activity was studied using the standard Trx/Trx reductase reduced NAD phosphate-coupled spectrophotometric assay as described in the supplemental Methods.
Animal studies
For the subcutaneous xenograft model, HL60 or NB4 cells were injected subcutaneously into female nude mice; then, the mice received an intratumoral injection of short hairpin RNA (shRNA) lentivirus. For the orthotopic xenograft model, HL60 cells or primary AML cells transduced with scrambled or shCDK2 lentivirus were injected into mice via the tail vein and the survival times of the mice were recorded (supplemental Methods).
Statistical analysis
See the supplemental Methods for details.
Results
CDK2 is specifically degraded during granulocytic differentiation in human AML cells
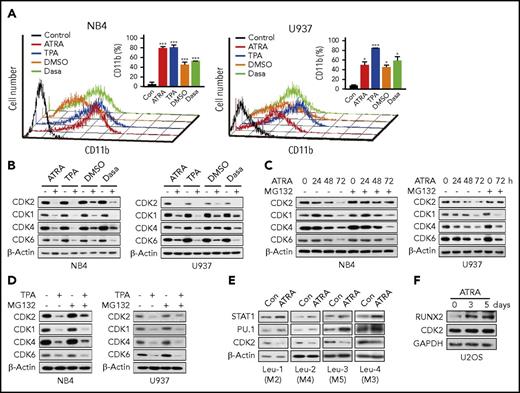
The protein levels of CDK are known to be relatively constant during the cell cycle, but whether they remain stable during the myeloid differentiation of AML cells is unknown. Because exit from the cell cycle is a necessary step in terminal differentiation, the degradation of a mitogenic factor is expected to occur in this process. Therefore, we first evaluated the protein stability of CDK in different myeloid differentiation cell models. Four chemicals were used to induce differentiation in AML cell lines (NB4 and U937) (Figure 1A). The results showed that CDK1, CDK2, CDK4, and CDK6 displayed distinct decreases during the differentiation process (Figure 1B). To further detect whether the decreases of these CDK were caused by degradation, the proteasome inhibitor MG132 was used. The ATRA-induced decrease in CDK2 was completely abolished by MG132, whereas the decreases of CDK1, CDK4, and CDK6 were not affected (Figure 1C). Similar results were obtained for 12-O-tetradecanoylphorbol 13-acetate (TPA) treatment (Figure 1D). In contrast, lysosomotropic autophagy inhibitors (CQ and 3-MA) failed to affect the ATRA-induced decrease in CDK2 (supplemental Figure 1A). Considering that CDK2 messenger RNA (mRNA) levels were rarely influenced by differentiation-inducing agents (supplemental Figure 1B), our results suggest that only CDK2 is specifically degraded during AML cell differentiation. Inversely, the protein levels of CDK2 remained constant during the cell cycle (supplemental Figure 1C-D), which provides further evidence of an association between CDK2 reduction and myeloid differentiation. Thus, these data indicate that CDK2 degradation is a specific consequence of myeloid differentiation in AML cells.
CDK2 is particularly degraded by the proteasome during AML cellular differentiation. (A) Effect of ATRA, TPA, DMSO, and Dasa on the cellular differentiation of NB4 and U937 cells, as assessed by CD11b expression. NB4 and U937 cells were treated with 0.1 or 1 µM ATRA for 72 hours, 10 ng/mL TPA for 24 hours, 1% DMSO for 120 hours, and 5 or 10 µM Dasa for 72 hours, respectively. (B-D) Protein expression levels of different CDK (CDK1, CDK2, CDK4, and CDK6) in NB4 and U937 cells after treatments, as evaluated by western blotting. (B) Both cells were treated as indicated in panel A. (C) NB4 and U937 cells were treated with 0.1 or 1 µM ATRA for the indicated times in the presence of vehicle or 1 μM MG132 for the final 8 hours of incubation. (D) Both cells were treated with 10 ng/mL TPA for 24 hours in the presence of vehicle or 1 μM MG132 for the final 8 hours of incubation. (E) Western blotting of STAT1, PU.1, and CDK2 in primary patient blasts (Leu-1-4) treated with 10 µM ATRA for 7 days. (F) Western blotting of RUNX2 and CDK2 in human osteosarcoma U2OS cells incubated with 5 µM ATRA for the indicated times. *P < .05; ***P < .001 vs control. Data are shown as mean ± standard deviation (SD), n = 3. Other data are representative of at least 3 individual experiments and 1 representative image is shown. Con, control; DMSO, dimethyl sulfoxide.
CDK2 is particularly degraded by the proteasome during AML cellular differentiation. (A) Effect of ATRA, TPA, DMSO, and Dasa on the cellular differentiation of NB4 and U937 cells, as assessed by CD11b expression. NB4 and U937 cells were treated with 0.1 or 1 µM ATRA for 72 hours, 10 ng/mL TPA for 24 hours, 1% DMSO for 120 hours, and 5 or 10 µM Dasa for 72 hours, respectively. (B-D) Protein expression levels of different CDK (CDK1, CDK2, CDK4, and CDK6) in NB4 and U937 cells after treatments, as evaluated by western blotting. (B) Both cells were treated as indicated in panel A. (C) NB4 and U937 cells were treated with 0.1 or 1 µM ATRA for the indicated times in the presence of vehicle or 1 μM MG132 for the final 8 hours of incubation. (D) Both cells were treated with 10 ng/mL TPA for 24 hours in the presence of vehicle or 1 μM MG132 for the final 8 hours of incubation. (E) Western blotting of STAT1, PU.1, and CDK2 in primary patient blasts (Leu-1-4) treated with 10 µM ATRA for 7 days. (F) Western blotting of RUNX2 and CDK2 in human osteosarcoma U2OS cells incubated with 5 µM ATRA for the indicated times. *P < .05; ***P < .001 vs control. Data are shown as mean ± standard deviation (SD), n = 3. Other data are representative of at least 3 individual experiments and 1 representative image is shown. Con, control; DMSO, dimethyl sulfoxide.
Mirroring the results in the cell lines, CDK2 protein rapidly turned over in response to ATRA in 4 primary AML samples (M2, M3, M4, and M5); PU.1 and STAT1 were also upregulated (Figure 1E). In contrast, the CDK2 levels remained constant in differentiated osteosarcoma U2OS cells (Figure 1F). Our results demonstrate the specificity of the decrease in CDK2 protein during granulocytic differentiation in AML cells.
CDK2 is a target for ubiquitin-mediated degradation both in vivo and in vitro
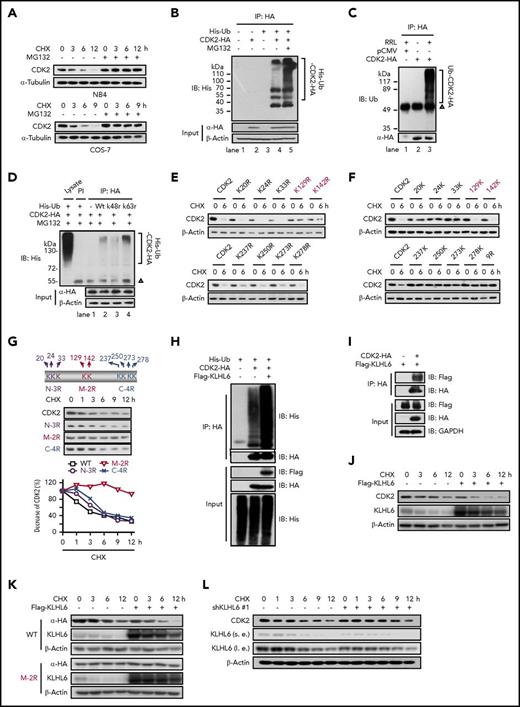
To further confirm that CDK2 is a substrate for ubiquitination, cycloheximide (CHX) was used to block new protein synthesis. CDK2 was unstable in the presence of CHX, whereas the turnover of CDK2 was blocked by MG132 (Figure 2A). CDK2 was also polyubiquitinated in vivo, which was further enhanced by MG132 (Figure 2B). The robust ubiquitination of HA-CDK2 (Figure 2C) and recombinant CDK2 (supplemental Figure 2A) was also observed in a cell-free system using rabbit reticulocyte lysate (RRL). Because lysine (K) residues at positions 48 and 63 are the major sites of ubiquitin chain initiation,16 we next tested whether the polyubiquitination of CDK2 involved the ubiquitination of these 2 residues. Ubk48r, but not Ubwt or Ubk63r, greatly reduced CDK2 ubiquitination, indicating that CDK2 is ubiquitinated via K48 in ubiquitin (Figure 2D).
Mapping of the ubiquitination and degradation pattern of CDK2. (A) The stability of CDK2 protein was measured by western blotting. NB4 and COS-7 cells were cultured with 10 µg/mL CHX for indicated times in the presence of vehicle or MG132 (1 or 10 µM). (B) Ubiquitination of CDK2 in COS-7 cells. COS-7 cells were cotransfected with pCMV-CDK2-HA and pEBB-His-C1-Ub for 48 hours, and 10 μM MG132 was added for the final 8 hours of culture. Ubiquitination was determined by immunoprecipitation with HA followed by western blotting with His antibody. (C) The ubiquitination of CDK2 in the RRL cell-free system. Cell extracts from COS-7 cells transfected with the pCMV or pCMV-CDK2-HA plasmid were subjected to anti-HA immunoprecipitation, followed by incubation with or without RRL at 30°C for 2 hours. (D) The effect of k48r and k63r ubiquitin on the ubiquitination of CDK2 in COS-7 cells. Ubiquitination was determined by immunoprecipitation with HA followed by western blotting with His antibody. PI: preimmune IgG; Δ, IgG heavy chain. (E) The turnover rates of various CDK2 mutants whose single lysine (K) was replaced with arginine (R) were measured by western blotting. COS-7 cells transfected with different CDK2 mutants were cultured with 10 µg/mL CHX for 6 hours. (F) The turnover rates of various CDK2 mutants carrying only 1 of the lysine as indicated. CDK2-9R is a mutant whose 9 lysine (Lys-20, Lys-24, Lys-33, Lys-129, Lys-142, Lys-237, Lys-250, Lys-273, and Lys-287) were replaced with arginine. These 9 mutants were derived from CDK2-9R by adding back individual lysine as indicated. (G) The decreased rates of various CDK2 mutants carrying 2 to 3 KR replacements. Diagrams of WT CDK2 with predicted ubiquitination sites and various CDK2 mutant constructs are shown. (H) The effect of KLHL6 on the ubiquitination of CDK2. HeLa cells were overexpressed with His-Ub, CDK2-HA, and Flag-KLHL6 for 48 hours, and then ubiquitination was determined by immunoprecipitation with HA followed by western blotting with His antibody. (I) The interaction between CDK2 and KLHL6 was detected by immunoprecipitation. HeLa cells were overexpressed with CDK2-HA and Flag-KLHL6 for 48 hours. After immunoprecipitation with HA their interaction were detected by western blotting with Flag antibody. (J) The effect of KLHL6 on the degradation of endogenous CDK2 protein as determined by western blotting. HeLa cells were overexpressed with Flag-KLHL6 followed with treatment with 10 μg/mL CHX for the indicated times. (K) The effect of KLHL6 on the degradation of WT CDK2 and CDK2 (M-2R) mutant. HeLa cells cotransfected with CDK2-HA (WT or M-2R) and Flag-KLHL6 plasmids were treated with 10 μg/mL CHX for the indicated times, and the exogenous CDK2 levels were determined by western blotting with HA antibody. (L) The effect of shKLHL6 on the degradation of endogenous CDK2 protein. HeLa cells were transfected with shKLHL6 #1 plasmids followed by CHX treatment of the indicated times, and the protein levels of CDK2 were determined by western blotting. HA, hemagglutinin; IP, immunoprecipitation; l.e., long exposure; s.e., short exposure. Data are representative of at least 3 individual experiments; 1 representative image is shown.
Mapping of the ubiquitination and degradation pattern of CDK2. (A) The stability of CDK2 protein was measured by western blotting. NB4 and COS-7 cells were cultured with 10 µg/mL CHX for indicated times in the presence of vehicle or MG132 (1 or 10 µM). (B) Ubiquitination of CDK2 in COS-7 cells. COS-7 cells were cotransfected with pCMV-CDK2-HA and pEBB-His-C1-Ub for 48 hours, and 10 μM MG132 was added for the final 8 hours of culture. Ubiquitination was determined by immunoprecipitation with HA followed by western blotting with His antibody. (C) The ubiquitination of CDK2 in the RRL cell-free system. Cell extracts from COS-7 cells transfected with the pCMV or pCMV-CDK2-HA plasmid were subjected to anti-HA immunoprecipitation, followed by incubation with or without RRL at 30°C for 2 hours. (D) The effect of k48r and k63r ubiquitin on the ubiquitination of CDK2 in COS-7 cells. Ubiquitination was determined by immunoprecipitation with HA followed by western blotting with His antibody. PI: preimmune IgG; Δ, IgG heavy chain. (E) The turnover rates of various CDK2 mutants whose single lysine (K) was replaced with arginine (R) were measured by western blotting. COS-7 cells transfected with different CDK2 mutants were cultured with 10 µg/mL CHX for 6 hours. (F) The turnover rates of various CDK2 mutants carrying only 1 of the lysine as indicated. CDK2-9R is a mutant whose 9 lysine (Lys-20, Lys-24, Lys-33, Lys-129, Lys-142, Lys-237, Lys-250, Lys-273, and Lys-287) were replaced with arginine. These 9 mutants were derived from CDK2-9R by adding back individual lysine as indicated. (G) The decreased rates of various CDK2 mutants carrying 2 to 3 KR replacements. Diagrams of WT CDK2 with predicted ubiquitination sites and various CDK2 mutant constructs are shown. (H) The effect of KLHL6 on the ubiquitination of CDK2. HeLa cells were overexpressed with His-Ub, CDK2-HA, and Flag-KLHL6 for 48 hours, and then ubiquitination was determined by immunoprecipitation with HA followed by western blotting with His antibody. (I) The interaction between CDK2 and KLHL6 was detected by immunoprecipitation. HeLa cells were overexpressed with CDK2-HA and Flag-KLHL6 for 48 hours. After immunoprecipitation with HA their interaction were detected by western blotting with Flag antibody. (J) The effect of KLHL6 on the degradation of endogenous CDK2 protein as determined by western blotting. HeLa cells were overexpressed with Flag-KLHL6 followed with treatment with 10 μg/mL CHX for the indicated times. (K) The effect of KLHL6 on the degradation of WT CDK2 and CDK2 (M-2R) mutant. HeLa cells cotransfected with CDK2-HA (WT or M-2R) and Flag-KLHL6 plasmids were treated with 10 μg/mL CHX for the indicated times, and the exogenous CDK2 levels were determined by western blotting with HA antibody. (L) The effect of shKLHL6 on the degradation of endogenous CDK2 protein. HeLa cells were transfected with shKLHL6 #1 plasmids followed by CHX treatment of the indicated times, and the protein levels of CDK2 were determined by western blotting. HA, hemagglutinin; IP, immunoprecipitation; l.e., long exposure; s.e., short exposure. Data are representative of at least 3 individual experiments; 1 representative image is shown.
Because CDK2 possesses 21 lysines, including 9 potential ubiquitination sites (K20, K24, K33, K129, K142, K237, K250, K273, and K287),17 we sought to define the lysine(s) required for CDK2 degradation. Analysis of a single lysyl replacement revealed that the turnover of K129R and K142R mutants was clearly impaired but not completely blocked (Figure 2E). Next, an “adding-back” approach was used; of the 9 mutants, 129K and 142K were consistently able to sustain the degradation of CDK2 (Figure 2F). Moreover, for CDK2 in which 2 of the 9 lysines were replaced (middle domain, M-2R), the turnover defect was substantially augmented compared with any single mutants or other 2 domain mutants (N-3R and C-4R) (Figure 2G). Thus, these results indicate that K129 and K142 are the preferential ubiquitination sites of CDK2.
KLHL6 is the ubiquitin E3 ligase that mediates the degradation of CDK2
Next, we sought to identify the ubiquitin E3 ligase responsible for the ubiquitination and degradation of CDK2 using Y2H screening.18 Human CDK2 was used as the bait to screen potential CDK2-interacting proteins from a Y2H prey library containing open reading frames encoding >400 putative ubiquitin ligases or their substrate binding subunits.15 Positive colonies were selected and harbored 10 prey vectors separately expressing TRIM10, ASB7, KCTD13, ZBTB20, PCGF2, RNF12, KLHL6, FBXO32, FBXW2, and RNF148. The in vivo ubiquitination assay results illustrated that only KLHL6 promoted the accumulation of polyubiquitinated CDK2 (supplemental Figure 2B; Figure 2H). Furthermore, the KLHL6-CDK2 interaction was confirmed by coimmunoprecipitation (Figure 2I). Next, we manipulated the KLHL6 levels and determined the stability of CDK2 protein. The results showed that the overexpression of KLHL6 enhanced the degradation of endogenous CDK2 (Figure 2J) and exogenous wild-type (WT) CDK2, but not a ubiquitination-deficient CDK2 mutant (M-2R) (Figure 2K). In contrast, the depletion of KLHL6 prolonged the half-life of endogenous CDK2 protein (Figure 2L; supplemental Figure 2C). These results indicate that KLHL6 functions as a ubiquitin E3 ligase for the CDK2 protein.
Depletion of CDK2 drives granulocytic differentiation in human AML cells
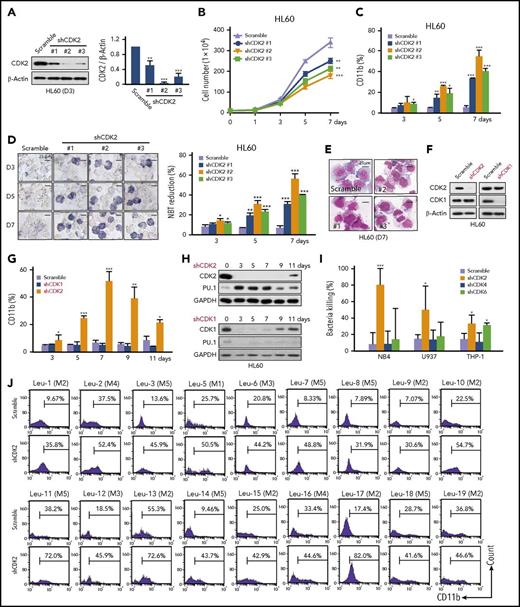
To further explore the effect of CDK2 on AML cell differentiation, 3 shRNA targeting CDK2 were introduced into HL60 cells (Figure 3A). All 3 shRNA could clearly inhibit cell proliferation (Figure 3B) and increase CD11b expression (Figure 3C) and NBT reduction activity (Figure 3D) in HL60 cells in a time-dependent manner. Moreover, CDK2-depleted cells had a mature morphology (Figure 3E). Similar differentiation effects were also obtained in NB4 and THP-1 cells (supplemental Figure 3). Taken together, these results clearly show that the depletion of CDK2 drives myeloid differentiation in AML cells.
Loss of CDK2 induces the differentiation of AML cells. (A) The silencing efficiency of different shRNA (#1, #2, and #3) against CDK2 in HL60 cells. The protein levels of CDK2 were measured by western blotting. (B-E) Cell differentiation analysis of HL60 cells infected with lentivirus-shCDK2s for the indicated times. (B) Cell proliferation assay. (C) CD11b expression. (D) NBT-reducing activity. (E) Cell morphological analysis after Wright-Giemsa staining. (F) The protein expression levels of CDK1 and CDK2 in HL60 cells infected with lentivirus-shCDK2 or lentivirus-shCDK1 for 72 hours. (G) CD11b expression in HL60 cells transduced with different lentiviruses for the indicated times. (H) Western blotting analysis of CDK2/1 and PU.1 expression in HL60 cells transduced with different lentiviruses for the indicated times. (I) Bacterial killing ability of THP-1, NB4, and U937 cells infected with different shRNA lentivirus (shCDK2 #2, shCDK4 #1, or shCDK6 #2) for 7 days. Clearance efficiency was determined from the numbers of viable bacteria recovered from the intracellular compartment after infection. S aureus were used to infect AML cells. (J) The differentiation of primary AML cells was assessed by CD11b expression. Primary AML samples collected from the peripheral blood of patients at diagnosis were infected with shCDK2 #2 lentivirus for 9 days. (A-D,G,I) *P < .05; **P < .01; ***P < .001 vs scramble. Data are shown as mean ± SD, n = 3. Other data are representative of at least 3 individual experiments; 1 representative image is shown. GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
Loss of CDK2 induces the differentiation of AML cells. (A) The silencing efficiency of different shRNA (#1, #2, and #3) against CDK2 in HL60 cells. The protein levels of CDK2 were measured by western blotting. (B-E) Cell differentiation analysis of HL60 cells infected with lentivirus-shCDK2s for the indicated times. (B) Cell proliferation assay. (C) CD11b expression. (D) NBT-reducing activity. (E) Cell morphological analysis after Wright-Giemsa staining. (F) The protein expression levels of CDK1 and CDK2 in HL60 cells infected with lentivirus-shCDK2 or lentivirus-shCDK1 for 72 hours. (G) CD11b expression in HL60 cells transduced with different lentiviruses for the indicated times. (H) Western blotting analysis of CDK2/1 and PU.1 expression in HL60 cells transduced with different lentiviruses for the indicated times. (I) Bacterial killing ability of THP-1, NB4, and U937 cells infected with different shRNA lentivirus (shCDK2 #2, shCDK4 #1, or shCDK6 #2) for 7 days. Clearance efficiency was determined from the numbers of viable bacteria recovered from the intracellular compartment after infection. S aureus were used to infect AML cells. (J) The differentiation of primary AML cells was assessed by CD11b expression. Primary AML samples collected from the peripheral blood of patients at diagnosis were infected with shCDK2 #2 lentivirus for 9 days. (A-D,G,I) *P < .05; **P < .01; ***P < .001 vs scramble. Data are shown as mean ± SD, n = 3. Other data are representative of at least 3 individual experiments; 1 representative image is shown. GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
Because a high degree of primary structural homology (86%) is observed between CDK2 and CDK1 and because inhibiting the CDK1 pathway has been previously reported to promote the myeloid maturation of FLT3ITD mutant leukemias,19 we further confirm the direct role of CDK2 and CDK1 in differentiation. Although both shCDK1 and shCDK2 could inhibit cell proliferation in AML cells (Figure 3F; supplemental Figure 4A), CDK2 but not CDK1 depletion upregulated CD11b and PU.1 expression levels in AML cells (Figure 3G-H; supplemental Figure 4B-C). These results strongly suggest that the knockdown of CDK2, but not CDK1, significantly induces myeloid differentiation in AML cells.
In addition to CDK1/2, CDK4/6 were also found to be downregulated during AML cell differentiation (Figure 1B), and it is reported that inhibiting CDK6 can overcome the blockage of differentiation associated with mixed-lineage leukemia (MLL)-rearranged AML20 ; thus, we examined the effect of CDK4/6 inhibition on differentiation in multiple subtypes of AML. In line with a previous study,20 shCDK4 had no effect on differentiation in 3 subtypes of AML cell lines (supplemental Figure 5A-B), whereas shCDK6 induced differentiation in MLL-rearranged THP-1 cells only and not in WT MLL-expressing cells (NB4 and U937) (supplemental Figure 5C-D). Consistently, only THP-1 cells are sensitive to pharmacologic CDK4/6 inhibitors (palbociclib and abemaciclib) (supplemental Figure 5E-G). However, our study found that shCDK2 could induce differentiation in all tested subtypes of AML, including THP-1, HL60, NB4, and U937 cells (Figure 3; supplemental Figures 3 and 4). Functional neutrophils are capable of killing bacteria; thus, we compare bactericidal activities between shCDK2- and shCDK4/6-induced differentiated AML cells. Interestingly, all AML cells treated with shCDK2 displayed significant bactericidal activities against Staphylococcus aureus infection, but not cells treated with shCDK4/6, suggesting that shCDK2 could induce more mature granules than shCDK4/6 (Figure 3I). Hence, compared with shCDK4/6, shCDK2 could induce more mature granulocytic cells in multiple subtypes of AML, including WT MLL-expressing cells.
Finally, the effect of shCDK2 was tested in primary AML samples. Although each sample demonstrated different specific profiles of the responses, CD11b expression was upregulated in all 18 samples (Figure 3J). Ten tested samples showed mature morphological changes and 6 tested samples showed the induction of STAT1, PU.1, and C/EBPβ expression (supplemental Figure 6A-B). In summary, the depletion of CDK2 relieves the differentiation blockade in both AML cell lines and primary samples.
Rescuing the transcription of the differentiation pathway is critical to the shCDK2-mediated differentiation process
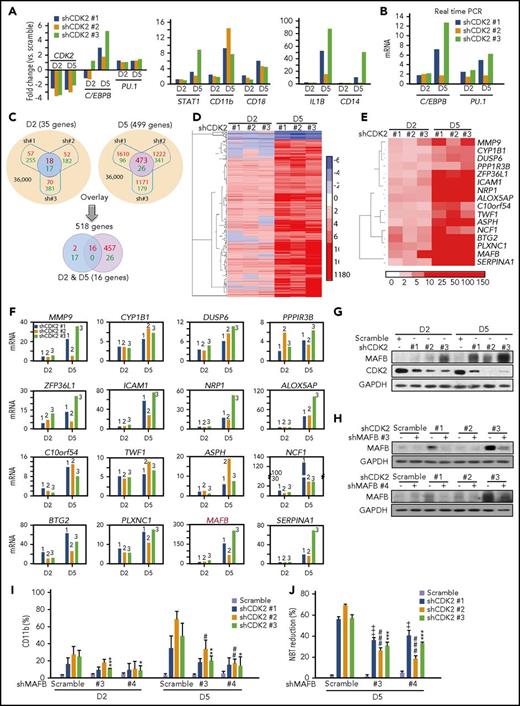
Cell differentiation is accompanied by the sustained reactivation of gene transcription. To confirm the effect of shCDK2 on differentiation, we performed gene expression analyses in NB4 cells infected with different lentivirus-shCDK2s at day 2 (initial differentiation stages with 6.58%, 10.3%, and 8.28% CD11b+ cells in shCDK2 #1, #2, and #3 groups) and day 5 (mature differentiation stages with 38.4%, 70.7%, and 51.3% CD11b+ cells in shCDK2 #1, #2, and #3 groups). As expected, the microarray results confirmed the differentiation phenotype of AML cells after CDK2-knockdown, because C/EBPB, PU.1, STAT1, CD11b, CD18, IL1B, and CD14 were distinctly increased in the shCDK2 groups (Figure 4A). Our real-time polymerase chain reaction (PCR) results further verified the upregulation of C/EBPB and PU.1 genes (Figure 4B).
Genome changes in AML cells following CDK2 depletion. (A-F) Microarray analysis of the gene expression changes in NB4 cells infected with lentivirus-shCDK2 (#1, #2, and #3) for 2 and 5 days. NB4 cells were subjected to GeneChip PrimerView Human Gene Expression Array for gene expression analyzes. (A) The mRNA changes of CDK2 and differentiation marker genes (C/EBPB, PU.1, STAT1, CD11b, CD18, IL1B, and CD14) generated from microarray analysis. (B) Validation of the mRNA changes of C/EBPB and PU.1 by real-time PCR. (C) Schematic representation of comparing gene expression profiles in NB4 cells. (Top) Overlapped smaller circles reflect the shared genes induced by different lentivirus-shCDK2s on day 2 (left; blue) and day 5 (right; purple), respectively. Top left, 35 genes changed on day 2 (up: 18 vs down: 17). Top right, 499 genes changed on day 5 (up: 473 vs down: 26). (Bottom) Overlapped smaller oval reflect the shared 16 genes induced on both day 2 and day 5. Red numbers in circles reflect upregulation, while green numbers reflect downregulation. (D) Heat map display of hierarchical clustering of overlapped genes sorted from top panel, (C). A total of 518 genes whose expression changed more than twofold on day 2 or day 5 were clustered. (E) (C) Heat map display of hierarchical clustering of overlapped 16 genes sorted from bottom panel. A total of 16 genes whose expression changed more than twofold both on days 2 and 5 were clustered. (F) Validation of 16 upregulated genes in (E) by real-time PCR. (G) The protein expression levels of MAFB and CDK2 in NB4 cells infected with lentivirus-shCDK2s for 2 or 5 days. (H-J) The effect of MAFB depletion on shCDK2-induced differentiation in NB4 cells. NB4 cells were infected with lentivirus-shMAFB (#3 and #4) for 1 day, followed by infection with lentivirus-shCDK2s for another 2 or 5 days. (H) Silencing efficiency of different shMAFBs. MAFB protein levels were evaluated by western blotting. (I) Percentage of CD11b expression. (J) NBT-reducing activity. (I-J) ++, P < .01; +++, P < .001; shMAFB vs scramble in the shCDK2 #1 group. #P < .05; ##P < .05; ###P < .001; shMAFB vs scramble in the shCDK2 #2 group. *P < .05; **P < .05; ***P < .001; shMAFB vs scramble in the shCDK2 #3 group. Data are shown as mean ± SD, n = 3. Other data are representative of at least 3 individual experiments; 1 representative image is shown.
Genome changes in AML cells following CDK2 depletion. (A-F) Microarray analysis of the gene expression changes in NB4 cells infected with lentivirus-shCDK2 (#1, #2, and #3) for 2 and 5 days. NB4 cells were subjected to GeneChip PrimerView Human Gene Expression Array for gene expression analyzes. (A) The mRNA changes of CDK2 and differentiation marker genes (C/EBPB, PU.1, STAT1, CD11b, CD18, IL1B, and CD14) generated from microarray analysis. (B) Validation of the mRNA changes of C/EBPB and PU.1 by real-time PCR. (C) Schematic representation of comparing gene expression profiles in NB4 cells. (Top) Overlapped smaller circles reflect the shared genes induced by different lentivirus-shCDK2s on day 2 (left; blue) and day 5 (right; purple), respectively. Top left, 35 genes changed on day 2 (up: 18 vs down: 17). Top right, 499 genes changed on day 5 (up: 473 vs down: 26). (Bottom) Overlapped smaller oval reflect the shared 16 genes induced on both day 2 and day 5. Red numbers in circles reflect upregulation, while green numbers reflect downregulation. (D) Heat map display of hierarchical clustering of overlapped genes sorted from top panel, (C). A total of 518 genes whose expression changed more than twofold on day 2 or day 5 were clustered. (E) (C) Heat map display of hierarchical clustering of overlapped 16 genes sorted from bottom panel. A total of 16 genes whose expression changed more than twofold both on days 2 and 5 were clustered. (F) Validation of 16 upregulated genes in (E) by real-time PCR. (G) The protein expression levels of MAFB and CDK2 in NB4 cells infected with lentivirus-shCDK2s for 2 or 5 days. (H-J) The effect of MAFB depletion on shCDK2-induced differentiation in NB4 cells. NB4 cells were infected with lentivirus-shMAFB (#3 and #4) for 1 day, followed by infection with lentivirus-shCDK2s for another 2 or 5 days. (H) Silencing efficiency of different shMAFBs. MAFB protein levels were evaluated by western blotting. (I) Percentage of CD11b expression. (J) NBT-reducing activity. (I-J) ++, P < .01; +++, P < .001; shMAFB vs scramble in the shCDK2 #1 group. #P < .05; ##P < .05; ###P < .001; shMAFB vs scramble in the shCDK2 #2 group. *P < .05; **P < .05; ***P < .001; shMAFB vs scramble in the shCDK2 #3 group. Data are shown as mean ± SD, n = 3. Other data are representative of at least 3 individual experiments; 1 representative image is shown.
Genes up- or downregulated more than twofold in all 3 shCDK2 groups at the 2- and/or 5-day time points were selected (Figure 4C; supplemental Table 1). Of these 36 000 transcripts, 35 and 499 genes displayed significant changes at days 2 and 5, respectively. These 518 altered genes changed on day 2 or day 5 were visualized with TreeView, and the majority (91.7%) were identified to have upregulated expression (Figure 4D). Interestingly, when these 2 groups of genes (days 2 and 5) were overlaid, only 16 upregulated genes were identified at both time points, and no genes were downregulated (Figure 4C,E). Therefore, these data indicate that CDK2 knockdown might have an activation effect on the transcription of the differentiation-associated genes.
We further verified the mRNA levels of these 16 genes by real-time PCR and found that MAFB increased most dramatically (Figure 4F). Considering that MAFB is reported to play an essential role in early myeloid and monocytic differentiation,21 we were encouraged to investigate whether MAFB is involved in shCDK2-induced differentiation. Similar to what we observed at the mRNA level, the protein expression of MAFB was obviously upregulated in shCDK2-transduced NB4 cells in a time-dependent manner (Figure 4G).
We also chose 2 shRNAs to develop stable MAFB-knockdown NB4 cells (Figure 4H). MAFB depletion had no effect on CD11b expression, whereas CDK2 depletion upregulated CD11b expression at both days 2 and 5 (Figure 4I). Moreover, shMAFB significantly reduced CD11b+ cells in CDK2-depleted cells, suggesting that MAFB silencing abolished the ability of shCDK2 to trigger differentiation. Similar observations were also found with the NBT reduction assay (Figure 4J). Taken together, these results clearly indicate that CDK2 depletion stimulates granulocytic differentiation by relieving the transcriptional blockage of MAFB, C/EBPB, PU.1, and other target genes.
Inhibition of CDK2 kinase activity relieves the blockage of granulocytic differentiation in AML cells without HSC cytotoxicity
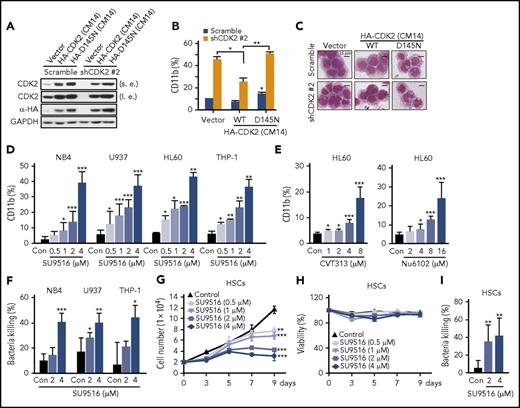
To test whether the differentiation caused by shCDK2 was dependent upon its kinase activity, we first reexpressed CDK2-CM14 (a shCDK2-#2-resistant mutant carrying synonymous mutations in its targeting sites) in CDK2-knockdown AML cells. As expected, shCDK2 was sufficient to induce AML cell differentiation, whereas the differentiation effect was clearly inhibited by reexpressing WT CDK2 (Figure 5A-C). In contrast, the overexpression of the dominant-negative mutant CDK2-D145N (CM14) failed to rescue the cells from differentiation. This result indicates that the kinase activity of CDK2 is required for its differentiation-blocking function.
Inhibition of CDK2 kinase activity relieves granulocytic differentiation of AML cells without HSC cytotoxicity. (A) The protein levels of CDK2 and HA-CDK2 in shCDK2-transfected HL60 cells reexpressing CDK2 (CM14) or CDK2-D145N (CM14). (B-C) Differentiation analysis of shCDK2-transfected HL60 cells reexpressing CDK2 (CM14) and CDK2-D145N (CM14). (B) CD11b expression and (C) morphological changes. (D) CD11b expression in NB4, U937, HL60, and THP-1 cells treated with indicated concentrations of SU9516 for 72 hours. (E) CD11b expression in HL60 cells treated with indicated concentrations of CVT313 or Nu6102 for 72 hours. (F) Bacterial killing ability of NB4, U937, and THP-1 cells treated with indicated concentrations of SU9516 for 5 days. S aureus was used to infect AML cells. (G) Proliferation assay and (H) viability assay of HSC cells treated with SU9516 for indicated times. Cells were stained with trypan blue and counted every 2 days. (I) Bacterial killing ability of HSC cells treated with indicated concentrations of SU9516 for 7 days. S aureus was used to infect HSC. (B,D-G,I) *P < .05; **P < .01; ***P < .001 vs control or as indicated. Data are shown as mean ± SD, n = 3. Other data are representative of at least 3 individual experiments; 1 representative image is shown.
Inhibition of CDK2 kinase activity relieves granulocytic differentiation of AML cells without HSC cytotoxicity. (A) The protein levels of CDK2 and HA-CDK2 in shCDK2-transfected HL60 cells reexpressing CDK2 (CM14) or CDK2-D145N (CM14). (B-C) Differentiation analysis of shCDK2-transfected HL60 cells reexpressing CDK2 (CM14) and CDK2-D145N (CM14). (B) CD11b expression and (C) morphological changes. (D) CD11b expression in NB4, U937, HL60, and THP-1 cells treated with indicated concentrations of SU9516 for 72 hours. (E) CD11b expression in HL60 cells treated with indicated concentrations of CVT313 or Nu6102 for 72 hours. (F) Bacterial killing ability of NB4, U937, and THP-1 cells treated with indicated concentrations of SU9516 for 5 days. S aureus was used to infect AML cells. (G) Proliferation assay and (H) viability assay of HSC cells treated with SU9516 for indicated times. Cells were stained with trypan blue and counted every 2 days. (I) Bacterial killing ability of HSC cells treated with indicated concentrations of SU9516 for 7 days. S aureus was used to infect HSC. (B,D-G,I) *P < .05; **P < .01; ***P < .001 vs control or as indicated. Data are shown as mean ± SD, n = 3. Other data are representative of at least 3 individual experiments; 1 representative image is shown.
Although several molecules that inhibit CDK2 have been developed, no CDK2 inhibitors have yet been approved for clinical use.22 This discrepancy might reflect a failure to use CDK2 inhibitors as apoptotic agents for chemotherapeutics. We analyzed the possibility of using CDK2-specific inhibitors to induce AML cell differentiation. Because almost all CDK2 inhibitors could exert inhibitory effects against CDK1, we selected several inhibitors (SU9516, CVT313, and Nu6102) that displayed higher activity against CDK2 than CDK1 (supplemental Figure 7A). Consistent with shCDK2, SU9516 treatment resulted in increased CD11b expression levels and NBT reduction ability in all 4 AML cell lines without obvious apoptosis induction (Figure 5D; supplemental Figure 7B-C). Similar results were also obtained using 2 other CDK2 inhibitors (CVT313 and Nu6102; Figure 5E). We also determined whether there are synergistic effects of CDK2 inhibitors and ATRA on differentiation. As expected, all tested CDK2 inhibitors (SU9516, CVT313, and Nu6102) displayed synergistic differentiation effects when combined with ATRA in all 3 AML cell lines (supplemental Figure 7D-F) and a primary AML sample (supplemental Figure 7G-H). Furthermore, SU9516 enhanced the bactericidal activities of multiple subtypes of AML (Figure 5F). Thus, we show that both pharmacological CDK2 inhibitors and CDK2 knockdown have differentiation-inducing effects on AML cells.
To confirm that CDK2 could be an effective therapeutic target in AML, we evaluated the effect of CDK2 inhibition on normal human HSCs. SU9516 inhibited cell proliferation without inducing cytotoxicity in human hematopoietic stem cells (HSCs) (Figure 5G-H). Moreover, CDK2 inhibition also induced granulocytic differentiation in HSC as assessed by cell morphological analysis (supplemental Figure 7I) and bacterial killing ability (Figure 5I). Thus, these results support the hypothesis that targeting CDK2 may be a promising treatment of AML patients.
CDK2 directly targets and activates PRDX2 to arrest AML cell differentiation
To gain insight into the mechanism of CDK2 knockdown-induced differentiation, we first examined PML-RARα expression and RARα activation, which are the classical targets of ATRA.23,24 Unlike ATRA, shCDK2 neither affected RARα activity nor cleaved or degraded PML-RARα (supplemental Figure 8A-B). Moreover, the RARE-luciferase assay also showed that CDK2 knockdown failed to modulate the transcriptional activation of RARα (supplemental Figure 8C-D).
Next, we performed coimmunoprecipitation assays followed by liquid chromatography-tandem mass spectrometry analysis, and 68 proteins were identified to physically interact with CDK2 in all 3 AML cell lines (supplemental Table 2); these proteins included 3 important peroxiredoxins (PRDX1, PRDX2, and PRDX6), which belong to a family of small nonseleno peroxidases that catalyze the peroxide reduction of H2O2. Interestingly, our results showed that H2O2 accumulation was clearly induced by shCDK2 in AML cells (supplemental Figure 9A), and N-acetyl-cysteine, a reactive oxygen species scavenger, notably abrogated shCDK2-induced differentiation in AML cells (supplemental Figure 9B-D). Given that (1) PRDX inhibition may contribute to AML differentiation,25 (2) H2O2 is an important second messenger for differentiation,26,27 (3) our data demonstrate that H2O2 accumulation contributes to shCDK2-induced differentiation, and (4) our liquid chromatography-tandem mass spectrometry data indicate an interaction between PRDX and CDK2, we proposed that CDK2 may target and activate PRDX to arrest AML cell differentiation.
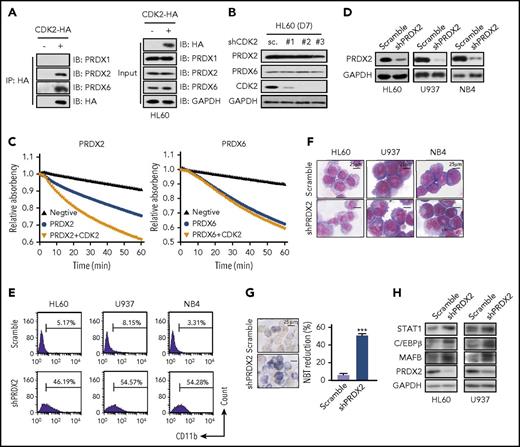
To confirm this hypothesis, we first validated the interaction between CDK2 and these PRDX. Our results revealed that PRDX1, PRDX2, and PRDX6 were expressed in AML cells, but only PRDX2 and PRDX6, not PRDX1, were pulled down by CDK2 (Figure 6A). Although CDK2 did not affect PRDX2 and PRDX6 expression levels in AML cells (Figure 6B), we found that rCDK2 effectively increased the peroxidase activity of rPRDX2, but not PRDX6 (Figure 6C). These results indicate that PRDX2 is a target of CDK2. Interestingly, when we knocked down PRDX2 expression in AML cells, increased CD11b expression levels were observed (Figure 6D-E). Furthermore, according to the morphological alterations (Figure 6F), NBT reduction (Figure 6G) and differentiation-related protein expression (Figure 6H), PRDX2-knockdown also induced differentiation in AML cells. Taken together, these data strongly support the hypothesis that the knockdown of CDK2 has a profound inhibition effect on the peroxidase activity of PRDX2, which leads to the accumulation of intracellular H2O2 levels and contributes to differentiation in AML cells.
CDK2 arrests AML cell differentiation by directly targeting and activating PRDX2. (A) The interaction between HA-CDK2 and PRDX1/2/6 was detected by immunoprecipitation. HL60 cells were transfected with CDK2-HA plasmid for 3 days. After immunoprecipitation with HA, their interaction were detected by western blotting with PRDX1/2/6 antibodies. (B) Western blotting of PRDX2/6 and CDK2 in shCDK2-transduced HL60 cells. (C) The effect of CDK2 on the peroxidase activities of PRDX2/6. The recombinant PRDX2/6 protein was incubated with recombinant CDK2 for 1 hour and peroxidase activity was monitored for 60 minutes. (D) Silencing efficiency of shRNA against PRDX2 in AML cells (HL60, U937, and NB4). (E-G) Cell differentiation analysis of AML cells infected with lentivirus-shPRDX2 for 7 days. (E) CD11b expression. (F) Cell morphological analysis after Wright-Giemsa staining. (G) NBT-reducing activity in HL60 cells. (H) Differentiation-related protein expression in AML cells infected with lentivirus-shPRDX2. The protein levels of STAT1, C/EBPβ, MAFB, and PRDX2 were measured by western blotting. (G) ***P < .001 vs scramble. Data are shown as mean ± SD, n = 3. Other data are representative of at least 3 individual experiments; 1 representative image is shown.
CDK2 arrests AML cell differentiation by directly targeting and activating PRDX2. (A) The interaction between HA-CDK2 and PRDX1/2/6 was detected by immunoprecipitation. HL60 cells were transfected with CDK2-HA plasmid for 3 days. After immunoprecipitation with HA, their interaction were detected by western blotting with PRDX1/2/6 antibodies. (B) Western blotting of PRDX2/6 and CDK2 in shCDK2-transduced HL60 cells. (C) The effect of CDK2 on the peroxidase activities of PRDX2/6. The recombinant PRDX2/6 protein was incubated with recombinant CDK2 for 1 hour and peroxidase activity was monitored for 60 minutes. (D) Silencing efficiency of shRNA against PRDX2 in AML cells (HL60, U937, and NB4). (E-G) Cell differentiation analysis of AML cells infected with lentivirus-shPRDX2 for 7 days. (E) CD11b expression. (F) Cell morphological analysis after Wright-Giemsa staining. (G) NBT-reducing activity in HL60 cells. (H) Differentiation-related protein expression in AML cells infected with lentivirus-shPRDX2. The protein levels of STAT1, C/EBPβ, MAFB, and PRDX2 were measured by western blotting. (G) ***P < .001 vs scramble. Data are shown as mean ± SD, n = 3. Other data are representative of at least 3 individual experiments; 1 representative image is shown.
Depletion of CDK2 arrests tumor growth, induces differentiation in human AML xenograft models, and prolongs the survival of NOD/SCID mice inoculated with AML cells
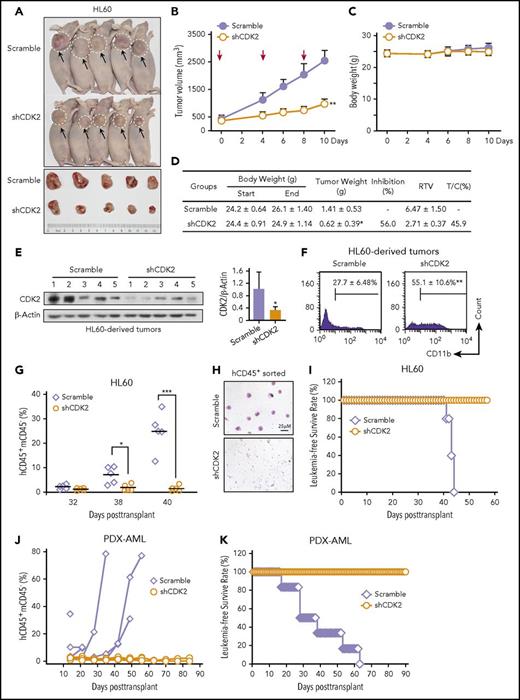
Finally, to test whether the differentiation-inducing effects of CDK2-knockdown could be reproduced in vivo, we evaluated the anti-tumor activity of CDK2-knockdown in HL60 and NB4 subcutaneous xenograft nude mouse models. When the tumors reached a volume >200 mm3, the mice received an intratumoral injection of lentivirus-scramble or lentivirus-shCDK2. As shown in Figure 7A-B, the average volume of tumors injected with a control lentivirus increased continuously, reaching an average volume at the end of the experiment of 2539 ± 386 mm3 compared with 969 ± 188 mm3 for the tumors injected with shCDK2 lentivirus (P < .01). Moreover, the shCDK2-lentivirus significantly reduced the tumor weight (56.0%) and displayed obvious therapeutic activity, as indicated by a relative tumor volume (RTV) T/C value of 45.9% (determined by RTVTreatment/RTVcontrol × 100) (Figure 7D). Importantly, no apparent weight loss was observed after shCDK2-lentivirus treatment (Figure 7C-D). After confirming the knockdown efficiency of the shCDK2 lentivirus (Figure 7E), we observed that 55.1% of the xenograft-derived HL60 cells were CD11b+ compared with the control group (27.7%, P < .01) (Figure 7F), indicating that the CDK2 depletion-mediated growth inhibition in the AML xenograft was a result of differentiation. Similar tumor suppression and differentiation-inducing effects of shCDK2 were noted in an NB4 xenograft mouse model (supplemental Figure 10A-B).
Inhibition of CDK2 effectively arrested tumor growth and prolonged the survival of AML-bearing mice. (A-F) Intratumoral therapy with the lentivirus encoding shRNA targeting CDK2 arrests tumor growth and induces human AML xenograft differentiation. (A) Images of mice and tumors in each group on day 10. (B) Tumor growth of HL60 xenografts. Tumor volume growth curves are expressed as the mean ± standard error, n = 5. Red arrow indicates the time of intratumoral injection. (C) Body weight of each group. (D) Effect of intratumoral therapy on mouse tumor weight and body weight at predose and postdose. Criteria for therapeutic activity: T/C (%), optimal growth inhibition <50 = effective. (E) Western blotting of CDK2 in each tumor derived from xenografts. (F) CD11b expression of cells derived from each tumor. (G-I) shCDK2 prolongs the survival of NOD/SCID mice inoculated with HL60 cells. (G) The population of human CD45+ and mouse CD45− (hCD45+mCD45−) leukemia cells in the peripheral blood of NOD/SCID mice are presented. (H) The fluorescence-activated cell sorting–purified hCD45+ leukemic blasts in the mouse peripheral blood displayed by blood smear analysis on day 40 after transplantation. (I) The survival times of NOD/SCID mice were recorded. (J-K) shCDK2 prolongs the survival of NSG mice inoculated with PDX-AML blasts (n = 6). (J) The population of hCD45+mCD45− leukemia cells in the peripheral blood of NSG mice were monitored weekly during the 3 months after transplant. (K) The survival times of NSG mice were recorded. (B,D-G) *P < .05, **P < .01, ***P < .001 vs scramble or as indicated. Data are shown as mean ± SD, n = 5 or 6. RTV, relative tumor volume; T/C, RTVtreatment/RTVcontrol × 100.
Inhibition of CDK2 effectively arrested tumor growth and prolonged the survival of AML-bearing mice. (A-F) Intratumoral therapy with the lentivirus encoding shRNA targeting CDK2 arrests tumor growth and induces human AML xenograft differentiation. (A) Images of mice and tumors in each group on day 10. (B) Tumor growth of HL60 xenografts. Tumor volume growth curves are expressed as the mean ± standard error, n = 5. Red arrow indicates the time of intratumoral injection. (C) Body weight of each group. (D) Effect of intratumoral therapy on mouse tumor weight and body weight at predose and postdose. Criteria for therapeutic activity: T/C (%), optimal growth inhibition <50 = effective. (E) Western blotting of CDK2 in each tumor derived from xenografts. (F) CD11b expression of cells derived from each tumor. (G-I) shCDK2 prolongs the survival of NOD/SCID mice inoculated with HL60 cells. (G) The population of human CD45+ and mouse CD45− (hCD45+mCD45−) leukemia cells in the peripheral blood of NOD/SCID mice are presented. (H) The fluorescence-activated cell sorting–purified hCD45+ leukemic blasts in the mouse peripheral blood displayed by blood smear analysis on day 40 after transplantation. (I) The survival times of NOD/SCID mice were recorded. (J-K) shCDK2 prolongs the survival of NSG mice inoculated with PDX-AML blasts (n = 6). (J) The population of hCD45+mCD45− leukemia cells in the peripheral blood of NSG mice were monitored weekly during the 3 months after transplant. (K) The survival times of NSG mice were recorded. (B,D-G) *P < .05, **P < .01, ***P < .001 vs scramble or as indicated. Data are shown as mean ± SD, n = 5 or 6. RTV, relative tumor volume; T/C, RTVtreatment/RTVcontrol × 100.
Next, we intravenously transplanted HL60-scrambled or HL60-shCDK2 cells into nonobese diabetic/severe combined immunodeficiency (NOD/SCID) mice to further assess the in vivo effects of shCDK2. The proportion of hCD45+mCD45− cells in the peripheral blood was analyzed dynamically to accurately reflect the overall leukemic burden. We found that the population of hCD45+mCD45− cells in the HL60-scrambled group increased dramatically on days 38 and 40 after the transplantation, whereas this population remained undetectable in the mice transplanted with HL60-shCDK2 cells (Figure 7G; supplemental Figure 10C), indicating that the shCDK2-lentivirus suppressed the engraftment of HL60 cells in vivo. Furthermore, the leukemic blasts in mouse peripheral blood were dramatically reduced after CDK2 knockdown according to May-Grünwald Giemsa staining with fluorescence-activated cell sorting–purified hCD45+ cells (Figure 7H). Overall survival was significantly better in mice transplanted with HL60-shCDK2 cells than in mice transplanted with scramble cells (Figure 7I), demonstrating that leukemic mice had a longer survival time following treatment with shCDK2. Similar results were also obtained in a PDX-AML xenograft model. shCDK2 treatment significantly decreased engraftment of primary human AML blasts and prolonged survival of leukemic NSG mice (Figure 7J-K). Collectively, these results are consistent with the notion that the inhibition of CDK2 induces AML cell differentiation and reduces the leukemic cell burden in both AML cell line and PDX-AML cells derived xenograft mouse models, further supporting that targeting CDK2 may be a promising treatment of differentiation in AML patients.
Discussion
In this study, we identify CDK2 as the key protein that is specifically degraded by the proteasome, and the depletion or pharmacological inhibition of CDK2 could relieve the blockage of differentiation in human AML cells. Thus, our data characterize a pivotal target for differentiation therapy.
During a normal cell cycle, the concentrations of CDK remain relatively constant.28 Unexpectedly, we demonstrate that CDK2 is degraded by the proteasome during granulocytic differentiation in AML cells. Such a decrease in CDK2 has been previously reported in other differentiation models.29,30 However, the mechanisms by which CDK2 is reduced during these processes remain unexplored. We confirmed the formation of ubiquitin and CDK2 by different systems and further identified the E3 ligase KLHL6 as a mediator of the ubiquitination and degradation of CDK2. KLHL proteins are known to be involved in the ubiquitination process,31,32 but specific roles for each family member have not yet been elucidated; thus, we described the first substrate of KLHL6.
Several lines of evidence indicate that CDK2 is not essential for cell proliferation; in particular, a variety of cancer cells are able to proliferate after CDK2 depletion,33 and CDK2 knockout mice are viable.8,9 Moreover, despite an intensive search over the past 2 decades for small molecules that target CDK2, no CDK2 inhibitors have been approved for clinical use.34 These observations might resolve the current intense debate regarding whether CDK2 is a useful cancer therapeutic target and clarify the other cell-type specific functions of CDK2 based on biochemical or cell biology studies. Interestingly, we identified a novel function of CDK2 as a differentiation-suppressing gene in leukemia cells. The depletion of CDK2 could markedly prolong the survival of mice inoculated with AML cells. This study provided intriguing data revealing CDK2 as a target of differentiation therapy.
Besides CDK2, CDK9, and CDK6 have been suggested as the therapeutic targets for AML, but their role in differentiation therapy of AML is not yet fully characterized. Previous studies suggest the effect of inhibiting CDK9 only block cell proliferation,35,36 and whether the inhibition of CDK9 could induce AML cells differentiation is unknown. Though the inhibiting of CDK6 can overcome the differentiation block, the evidence suggests that it specifically works on MLL-rearranged AML, which is only account for 5% to 10% of AML.20 It should be noted that the evolutionary expansion of the CDK family in mammals led to the division of CDKs into 3 cell-cycle–related subfamilies (CDK1, CDK4, and CDK5) and 5 transcriptional subfamilies (CDK7, CDK8, CDK9, CDK11, and CDK20).37,38 CDK2 belongs to CDK1 subfamily, which is only CDK essential for the cell cycle in mammals,9,10 suggesting its function different from other CDKs subfamily members. Importantly, inhibiting CDK2, but not its homolog CDK1/4/6, effectively induced granulocytic differentiation in 5 major subtypes of primary patient-derived AML samples. To our knowledge, there is no evidence so far support the idea that inhibition of CDK2 drives the therapeutic differentiation on 5 major subtypes of AMLs (M1-M5). Thus, it’s the first time to show that CDK2 is the ideal target for differentiation therapy in multiple subtypes of AML.
Because of the observation that various CDK2 inhibitors could promote the differentiation process in AML cells, our findings pioneer new possibilities for the use of CDK2 inhibitors in leukemia differentiation therapy and provide a rational basis for the design of more focused clinical trials. Cells that lack different combinations of CDKs (CDK2, CDK4, and CDK6) exited quiescence and proceed through the early phase of the cell cycle,39-41 thereby suggesting that CDK2 inhibitors will display limited toxicity; this outcome is also ideal for differentiation therapy.
Our findings reveal a previously unrecognized activity of CDK2 and raise questions regarding its functions as a differentiation regulator. It is possible that the depletion of CDK2 could affect PML-RARα, a leukemia-generating fusion protein.42,43 However, the stable expression of PML-RARα after CDK2 depletion prompted us to identify the potential targets of CDK2. Finally, we discovered that CDK2 could specifically upregulate PRDX2 peroxidase activity. However, the mechanisms underlying the CDK2-induced increase in PRDX2 activity warrant further investigation.
In summary, we have demonstrated for the first time that differentiation agents act in a novel manner to decrease CDK2 expression by stimulating the degradation of CDK2. The genetic or pharmacological inhibition of CDK2 can drive granulocytic differentiation in AML cells. Based on these newly achieved mechanistic insights, this study not only directs us toward the future application of CDK2 inhibitors but also provides fresh insights into the design of new therapies targeting differentiation in leukemia.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors acknowledge D. B. Kohn for providing lentivirus pRΔ8.9 and pMD.G plasmids and E. Burstein (University of Texas Southwestern Medical Center) for providing pEBB-His-C1-Ub plasmids.
This work was supported by the National Science Fund for Distinguished Young Scholars (81625024) and grants from National Natural Science Foundation of China (81273534 and 81473227).
Authorship
Contribution: M.Y. and X.S. performed experiments and analyzed data; M.Y., Q.H., and B.Y. conceived the study; H.J. and S.X. performed the ubiquitin study; Y.L., Y.C., and X.Q. performed the western blotting procedure; H.S. and G.W. collected samples and data from leukemia patients; J.C. conceived the experiments and helped organize the paper; M.Y., Q.H., and R.H. wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Qiaojun He, Institute of Pharmacology & Toxicology, College of Pharmaceutical Sciences, Zhejiang University, Room 427, Hangzhou, 310058, China; e-mail: qiaojunhe@zju.edu.cn; and Bo Yang, Institute of Pharmacology & Toxicology, College of Pharmaceutical Sciences, Zhejiang University, Room 427, Hangzhou, 310058, China; e-mail: yang924@zju.edu.cn.