In this issue of Blood, Battistello et al present results exploring inhibition of signaling across key nodes in the B-cell receptor (BCR) signaling pathway for Bruton tyrosine kinase (BTK)-sensitive and BTK-insensitive diffuse large B-cell lymphoma (DLBCL).1
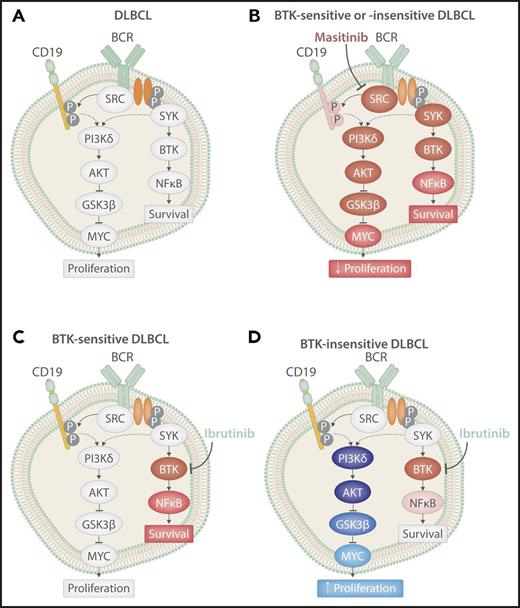
BCR signaling in DLBCL cells at baseline or after treatment. BCR signaling in DLBCL at baseline (A) after treatment with ibrutinib in a BTK-sensitive cell (B) or BTK-insensitive cell (D), or after treatment with the pan-SRC inhibitor masitinib (C). Red shading indicates inhibition of protein signaling, and blue shading indicates activation of protein signaling. The extent of shading corresponds with the extent of change. P, phosphorylation sites. Professional illustration by Somersault18:24.
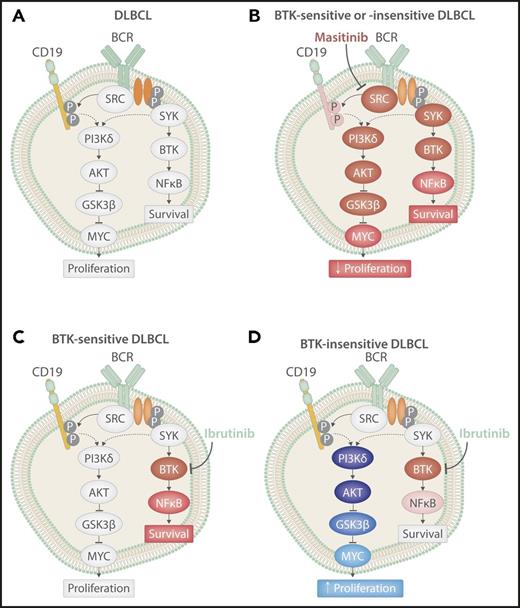
BCR signaling in DLBCL cells at baseline or after treatment. BCR signaling in DLBCL at baseline (A) after treatment with ibrutinib in a BTK-sensitive cell (B) or BTK-insensitive cell (D), or after treatment with the pan-SRC inhibitor masitinib (C). Red shading indicates inhibition of protein signaling, and blue shading indicates activation of protein signaling. The extent of shading corresponds with the extent of change. P, phosphorylation sites. Professional illustration by Somersault18:24.
DLBCL is an aggressive form of non-Hodgkin lymphoma with 2 major, molecularly distinct, subtypes, activated B-cell-like (ABC) and germinal center B-cell-like (GCB) DLBCL. These subtypes arise from different mechanisms and display differing signaling patterns. Furthermore, these subtypes have differential survival rates after treatment with traditional chemotherapy, with ABC-DLBCL having a substantially poorer prognosis. One key difference is the dependence on nuclear factor κB (NF-κB) activity for survival of ABC, but not GCB, DLBCL downstream of BTK in the BCR signaling pathway.2 These signaling differences translate to differences in response to targeted agents, exemplified by ibrutinib monotherapy, where 37% of patients with ABC-DLBCL but only 5% of patients with GCB-DLBCL had complete or partial responses in a study done by Wilson et al.3 Although BTK is a key node in the BCR pathway, ligation of the BCR promotes activation of multiple downstream targets, including BTK, CD19 (BCR coreceptor), and phosphoinositide 3-kinase (PI3K). Recently, GCB-DLBCL has been shown to use tonic BCR signaling (in contrast to antigen-dependent BCR signaling that occurs in ABC-DLBCL) with a strong dependence on spleen tyrosine kinase (SYK) and PI3K, suggesting that targeting alternative BCR nodes could be clinically beneficial.4 Despite significant biological differences and the requirement of cell-of-origin classification as part of DLBCL classification in the 2016 revised World Health Organization classification of lymphoid neoplasms, methods to determine subtypes remain a challenge,5 suggesting a benefit for more universal “targeted” agents for the treatment of DLBCL.
Battistello et al investigated the change in BCR signaling across important nodes in DLBCL patients, representing 4 GCB, 1 ABC, 2 double-hit lymphomas, and multiple well-described cells lines. Stimulation of the BCR pathway, by anti-BCR antibodies, led to increased activation of BTK, CD19, and glycogen synthase kinase 3 β (GSK3β) in a majority of tumors independent of subtype. Treatment with ibrutinib led to inhibition of BTK but not typically CD19 or GSK3β, again independent of subtype and sensitivity to BTK (see figure). Interestingly, despite similar changes in BTK activation levels, ibrutinib-resistant cell lines exhibited a significant upregulation of MYC upon ibrutinib treatment, whereas those sensitive to BTK inhibition downregulated MYC (see figure). This change in MYC expression corresponded to changes in proliferation in both cell lines and murine B-cell lymphomas resistant to ibrutinib, with an increase in MYC leading to more tumor proliferation. This finding is important because it suggests that failure to fully inhibit BCR signaling in BTK-insensitive DLBCL, regardless of subtype, could allow for a compensatory pathway to be upregulated leading to a more aggressive disease. Furthermore, it suggests that changes in expression of MYC could be used as a potential biomarker of response to ibrutinib in DLBCL, potentially allowing for the early determination of patients who will not benefit from treatment.
Given the activation of alternative BCR nodes (specifically PI3K) that are directly responsible for the observed MYC upregulation in cell lines that are resistant to ibrutinib, combination treatment with ibrutinib and idelalisib (PI3Kδ inhibitor) was evaluated. DLBCL cell lines insensitive to single-agent treatment became sensitive to the combination, demonstrating synergy to promote apoptosis and inhibit cell proliferation through dual targeting of BTK and PI3K. Although combination therapy may elicit better results, a phase 1 trial of single-agent idelalisib demonstrated no response in DLBCL.6 In contrast to single-agent inhibition of BTK or PI3K, which inhibits only 1 node in the BCR signaling pathway, inhibition of SRC-kinases prevents downstream propagation of BCR signaling across multiple nodes. Masitinib, a pan-SRC kinase inhibitor that targets lymphocyte-specific protein kinase, tyrosine-protein kinase lyn, tyrosine-protein kinase blk, and proto-oncogene tyrosine-protein kinase fyn (all members of the SRC kinase family) currently in phase 3 trials for amyotrophic lateral sclerosis, was demonstrated to be highly effective against DLBCL, with 83% of cell lines showing sensitivity to the drug. Furthermore, masitinib resulted in inhibition of BTK, CD19, GSK3β, and MYC, inducing apoptosis and inhibiting proliferation in vitro and in patient-derived xenografts regardless of their molecular subtype (see figure). This provides proof of concept for the possibility of a more “universal” yet targeted treatment of DLBCL patients, independent of their subtype.
Although these data points very nicely demonstrate the benefit of broader kinase inhibition across DLBCL subtypes, toxicity of such a treatment remains a concern. Battistello and colleagues found that masitinib was well tolerated in mice as determined by weight loss and lack of alopecia; however, no formal evaluation of other toxicities was described. Treatment with kinase inhibitors has displayed varying toxicities, with combination therapy typically displaying more toxicity than single agents. Most notably, patients receiving combination treatment with entospletinib (an SYK inhibitor) and idelalisib experienced high rates of pneumonitis, potentially suggesting that broad spectrum kinase inhibition may need to be carefully monitored.7
Despite the important biological differences between subtypes of DLBCL, R-CHOP (rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone) remains the gold standard of care8 ; however, novel therapies are very much needed for the ∼40% of patients who relapse after initial treatment or have refractory disease. Multiple drugs are currently under clinical investigation for DLBCL (with a focus on ABC-DLBCL), including alternative kinase inhibitors (targeting BTK, mammalian target of rapamycin, and PI3K), immunotherapies (including lenalidomide and chimeric antigen receptor T cells), and other targeted agents (such as venetoclax, a BCL-2 inhibitor).9 How SRC-kinase inhibitors such as masitinib will fit into this already packed playing field remains to be seen.
Conflict-of-interest disclosure: The author declares no competing financial interests.