TO THE EDITOR:
Age is known to be a strong negative predictor of survival in patients undergoing an allogeneic hematopoietic stem cell transplantation (HSCT) for severe acquired aplastic anemia (SAA), with higher mortality in patients >40 years of age,1 and this has been confirmed in several large studies.2-7
SAA can also be treated with immunosuppressive therapy (IST), with a lower risk of early complications, and first-line IST and bone marrow transplant have been compared in different age groups.5 Currently, the international guidelines recommend IST first line, older than the age of 408 or 50.9 The question is: are these age cutoff values still valid today? In previous studies, survival after transplantation improved from 48% (in the 1976-1980 cohort) to 66% (in the 1988-1992 cohort),4 and more recently from 61% to 76% in patients grafted before 1999 or between 1999 and 200910 : the latter study included both sibling (SIB) and unrelated donor (UD) grafts as well as children and adults. Improved outcome may have been the consequence of changes in graft-versus-host disease (GvHD) prophylaxis,4 as well as changes in the conditioning regimens,11-15 better donor selection, and a larger use of antithymocyte globulin (ATG).2,16 Nevertheless, for patients older than the age of 40, transplant-related mortality continued to be on the order of 50% in the 1999 to 2009 period. In more recent years, supportive care has further improved and may have reduced the risk of transplant-related complications.
We have thus compared the outcome of SAA patients older than the age of 40 years, transplanted in 2001 to 2009 (n = 329), with patients transplanted in 2010 to 2015 (n = 439). Clinical characteristics of patients are outlined in Table 1. The study was approved by the Internal Review Board of the Hematology Institute, Policlinico Gemelli, Rome, Italy. In the more recent period, patients were older, with more UDs; there was a greater use of ATG or alemtuzumab (CAMP), marrow, and fludarabine. The statistical analysis was performed with NCSS software (NCSS 11 Statistical Software−2016; NCSS, LLC, Kaysville, UT; ncss.com/software/ncss). Comparisons between transplant groups were carried out using the χ2 test for categorical variables and the nonparametric Mann-Whitney U test for continuous variables. Univariate and multivariate analyses were carried out using the Cox proportional hazard model. Actuarial survival was calculated according to Kaplan and Meier.
Combined primary and secondary graft failure (GF) was reported in 48 and 47 patients in the 2 time periods (14.5% vs 10.7%, P = .1). Primary GF was twice as frequent than secondary GF (8.2% vs 4.1%). Acute GvHD grade II to IV was comparable in the 2 periods (15% vs 11%, P = .1), whereas chronic GvHD was reduced from 31% to 25% (P = .01). Extensive chronic GvHD occurred in 10% and 15% of patients grafted from identical SIBs or UD (P = .01).
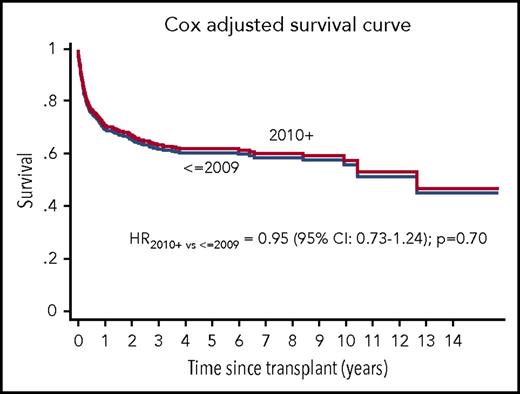
The 5-year survival of patients grafted in 2001 to 2009 or 2010 to 2015 was 61% vs 58% (P = .7). In univariate analysis, significant predictors of survival were patient’s age, the use of ATG or CAMP, and center experience. The 5-year overall survival of patients aged 40 to 49 years, 50 to 59 years, and >60 years was 67%, 58%, and 45%, respectively (P < .0001). When patients receiving either CAMP or ATG (n = 564) were compared with patients not receiving either (n = 161), the difference in survival was 63% vs 48% (P < .0001). Survival of patients grafted in centers with >3 patients in this study did significantly better than patients grafted in centers with 1 to 3 patients in the study (65% vs 48%, P = .0001). This difference was maintained in the age group 40 to 49 years (73% vs 54%, P = .001), in the age group 50 to 59 years (66% vs 42%, P = .002), but not in patients >60 years (46% vs 44%, P = .7). When stratifying conditioning regimens according to the use of fludarabine, there was no significant effect on survival: for patients receiving UD grafts, the 5-year survival was 58% for fludarabine-based vs 51% for non-fludarabine-based regimens (P = .3). For SIB grafts, the 5-year survival was 65% vs 62% (P = .5). Infections remain the leading cause of death in both transplant eras (16% and 19%, respectively), followed by GvHD (5% and 3%) and organ toxicity (7% and 4%).
A multivariate analysis confirmed the lack of improved survival in 2010 to 2015, as compared with 2001 to 2009 (hazard ratio [HR] 0.95, 95% confidence interval [CI] 0.73-1.24; P = .7), as shown in Figure 1, adjusted for patient’s age, the use of either ATG or CAMP in the conditioning regimen, center experience, and donor type (SIB vs UD). Other significant variables were the following: patient’s age 50 to 59 vs 40 to 49 years (HR 1.16, 95% CI 0.87-1.53; P = .2), >60 vs 40 to 49 years (HR 1.89, 95% CI 1.32-2.55; P = .00001); the use of ATG or CAMP vs no ATG nor CAMP (HR 0.59, 95% CI 0.44-0.77; P = .0002); centers with >3 transplants vs centers with 1 to 3 transplants (HR 0.60, 95% CI 0.47-0.77; P = .0001). Stem cell source and interval diagnosis-HSCT were not predictive in the Cox analysis.
Overall survival and transplant era. Survival of patients with acquired aplastic anemia, aged 40 years and older, allografted between 2001 and 2009 (<2009) (n = 329) or 2010 to 2015 (2010+) (n = 439), adjusted for patient's age, the use of either ATG or CAMP in the conditioning regimen, donor type, and center experience.
Overall survival and transplant era. Survival of patients with acquired aplastic anemia, aged 40 years and older, allografted between 2001 and 2009 (<2009) (n = 329) or 2010 to 2015 (2010+) (n = 439), adjusted for patient's age, the use of either ATG or CAMP in the conditioning regimen, donor type, and center experience.
We have shown in the present study that survival has remained unchanged in the past 15 years in patients with SAA, older than the age of 40 years, undergoing an allogeneic HSCT: this was true also when correcting for confounding variables such as patient’s age, donor type, in vivo T-cell depletion with ATG or CAMP, and center experience. Previous reports showing improved outcome in SAA patients7,13 included children and young adults, with an upper age limit at 55 years: it may be that improvement is more difficult to achieve in this older patient population. One single-center study shows no effect of age for SAA patients grafted from identical SIBs11 : our study is a multicenter registry-based analysis, which may better reflect real-life outcome. We did not see an effect of stem cell source: it should be said that the first study on stem cell source17 had failed to show a significant effect over the age of 20, and only a second study had shown a positive effect of BM, also above the age of 20, in all age groups.18 It could also be a question of numbers, because transplants under the age of 20 and 40 are far more frequent than >40 years of age. We were impressed with the center effect, suggesting that this rare disease should be treated in centers with expertise, because the difference in survival is on the order of 20%, up to the age of 60 years. GvHD prophylaxis is another crucial variable, with significant advantage when the program includes either ATG or CAMP. When combining the 3 positive predictors, age <60, experienced centers, and ATG/CAMP, the 5-year survival remains 72% and has not changed in the most recent period.
Limitations of this study include the arbitrary choice of the cutoff year of transplant, the fact that performance score and HSCT comorbidity index were not available, and the role HLA matching for UDs, which was not studied. Nevertheless, overall, allogeneic transplants for SAA over the age of 40 years continues to carry a significant risk of mortality, which has not been reduced in the current era, despite changes in conditioning regimens and donor type, also in patients receiving an HLA identical SIB transplant. This finding supports current guidelines, suggesting first-line immunosuppression for patients over the age of 40 years.
Acknowledgment
The authors thank the European Group for Blood and Marrow Transplantation (EBMT) centers for contributing their patients to the EBMT Working Party aplastic anemia database.
Authorship
Contribution: A.B. and S.G. designed the study; R.O. prepared the database; S.G. and A.B. wrote the manuscript; R.P.d.L., S.S., C.D., G.S., J.P., N.K., E.P., M.T.V.L., and A.B. contributed patients and reviewed the manuscript; and A.B., R.O., and A.S. ran the statistical analysis.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
A complete list of the members of the European Group for Blood and Marrow Transplantation Severe Aplastic Anemia Working Party appears in “Appendix.”
Correspondence: Andrea Bacigalupo, Istituto di Ematologia, Fondazione Policlinico Universitario Gemelli, Largo A Gemelli 9, 00168 Rome, Italy; e-mail: apbacigalupo@yahoo.com.
Appendix: study group members
The members of the European Group for Blood and Marrow Transplantation Severe Aplastic Anemia Working Party are: Carlo Dufour (Genoa, Italy), Régis Peffault de Latour (Paris, France), Antonio Risitano (Naples, Italy), Judith Marsh (London, United Kingdom), Andrea Bacigalupo (Rome, Italy), Maria Teresa Van Lint (Genoa, Italy), Jacob Passweg (Basel, Switzerland), Gerard Socie (Paris, France), Andree Tichelli (Basel, Switzerland), Hubert Schrezenmeier (Ulm, Germany), Britta Hochsmann (Ulm, Germany), Mahmoud Deeb Saeed M. Aljurf (Riyadh, Saudi Arabia), Stijn Halkes (Leiden, The Netherlands), Marc Bierings (Utrecht, The Netherlands), Sujit Samarasinghe (London, United Kingdom), Austin Kulesekararaj (London, United Kingdom), Simona Iacobelli (Rome, Italy), Cora Knol (Leiden, The Netherlands), Paul Bosman (Leiden, The Netherlands), Dirk Jan EIkema (Leiden, The Netherlands) Anija Van Biezen (Leiden, The Netherlands), Maurizio Miano e Francesca Fioredda (Genoa, Italy), Paul Miller (London, United Kingom), Alicia Rovò (Bern, Switzerland), Fabien Beir (Achen, France).