Abstract
Background: Relapsed/refractory MM (RRMM) patients remain highly unmet need. Chimeric antigen receptor (CAR) T cells targeting B-Cell Maturation Antigen (BCMA) in pre-clinical trials have displayed encouraging anti-tumor activity. Though CD19 is expressed only rarely on MM plasma cells (PC), rare CD19+ B cells can be identified in MM patients that are clonally related to the MM PC. These clonotypic B cells may exhibit properties of cancer stem cell. Thus, CD19 is also a potential therapeutic target in conjunction with other therapies that target MM PC. This study was designed to observe the safety and efficacy of combined infusion of CD19 and BCMA-Specific CART Cells for RRMM (NCT 03196414).
Methods: Human T cells were collected from auto/allo-logous peripheral blood mononuclear cells (PBSC) and cultured with an anti-CD3 monoclonal antibody to activate proliferation. The cells were transduced with recombinant lentiviral verctors which respectively contained the anti-BCMA or anti-CD19 single chain variable fragment (scFv), the cytoplasmic portion of the OX40 and CD28 costimulatory moiety, and the CD3z T-cell activation domain. This is the new third generation CAR technique applied in clinic. In this ongoing, RRMM patients (pts) receive 3 doses of 300mg/m2 of cyclophosphamide and 3 doses of 30mg/m2 of Fludarabine iv qd×3 days on day -5,-4 and -3 as lymphodepleting conditioning , then are infused into CART-19 (1×107/kg on day 0) and CART-BCMA cells as split-dose infusions (40% on day 1 and 60% on day 2). BCMA expression on MM cells and CART-related cytokine release syndrome (CRS) are analyzed by flow cytometry. Levels of CAR-transduced cells are also measured by qPCR using a transgene-specific primer/probe pair. Chimerism (short tandem repeats, STRs) was determined by donor/receptor microsatellite detection for patients with infusion of allogeneic CART cells. Responses are assessed by IMWG criteria. The median follow-up times was 5 (2 ∼; 20) weeks.
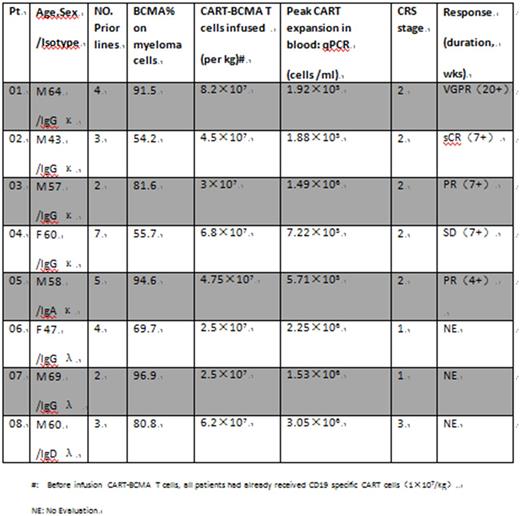
Results: To date, 8 pts have completed the CART cells infusion (cohort 1). Because pts 04 and 05 are invalid for previous autologous CART-BCMA immunotherapy, CART cells in this trial for these two pts were prepared from relative haploidentical donor peripheral blood T cells. The most common adverse event of CART therapy is acute CRS, which occurred in 8 patients (100%). High-grade CRS was associated with elevated levels of IL-6, IFNγ and IL-10. The cytokine levels increased significantly in two patients (pts 01 and 08) ,especially IL-6 up thousands of times from the baseline. But according to the criterias of CTCAE_V4.02, pt 01 was evaluated grade 2 of CRS and pt 08 was grade 3 due to abnormal renal and blood coagulation function. These two corresponding patients fully recovered within a week after tocilizumab (4mg/kg) treatment. Other outstanding toxicities to date include fatigue (n=8), cytopenia (n=8), anemia (n=6), coagulopathy (n=5), elevated bilirubin and AST(n=4), pulmonary edema (n=3) and pleural effusions and ascites (n=1). All patients did not need to use the vascular active drug. No neurologic complications and treatment related mortality (TRM) occurred in this cohort. There did not happend reactions of Graft-versus-host (GVH) and rejection of CART by host-versus-graft (HVD). STRs is both 2%-3% in pts 04 and 05.
Among the 8 infused patients, 5 patients were monitored for a period of more than 4 weeks. 100% (5/5) pts had prolonged cytopenias. 4 out of 5 cases acquired above effects of partial remission (PR), which may be eventually further improved. The three patients who were followed up for less than 4 weeks have an asymptomatic living. Pt 02 had progressive MM with extramedullary lesion persisted in his right psoas muscle mass, with gradually increasing. He received local injection of 0.5×107/kg CART-BCMA cells on day 2 into the tumor mass under ultrasound guidance. Miraculously, 4 weeks later, imaging revealed a complete disappearance of the tumor and free light chain levels in his peripheral blood decreased below the detection limit.
Conclusions: Combined administration of auto/allo-logous CART-19 and CART-BCMA cells can be manufactured from heavily-pretreated MM pts, and demonstrate promising in vivo expansion and clinical activity. Toxicities to date can be controlled and reversed, without TRM. Expanded accrual in cohort 1 is ongoing, with updated data to be presented at the meeting.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.