Abstract
The B-cell leukemia/lymphoma-2 (BCL-2) family of proteins governs the intrinsic pathway of mitochondrial apoptosis. Dysregulation of BCL-2 has long been known to be a crucial part of the pathophysiology of B-cell lymphomas; however, several early attempts to target this pathway therapeutically were unsuccessful because of toxicity, lack of efficacy, or both. Recently, a highly potent and selective oral BCL-2 antagonist, venetoclax, was approved in chronic lymphocytic leukemia, where it has proven to be highly active, even in patients with high-risk del(17p) disease. Venetoclax has also demonstrated efficacy in other B-cell non-Hodgkin lymphoma subtypes, in particular mantle cell lymphoma and follicular lymphoma. Here, I review the history of targeting BCL-2 in B-cell lymphomas, and I discuss recent data on venetoclax used as monotherapy and in combination with monoclonal antibodies, chemotherapy, and other novel agents. I also discuss how genomic and functional approaches such as BH3 profiling may allow us to prioritize novel-agent combinations for further study in clinical trials. These approaches may also help us to understand resistance mechanisms to BCL-2–selective therapy and how to overcome resistance. Finally, I provide my perspective on how to move BCL-2–directed therapies forward toward a goal of developing well-tolerated, time-limited combination regimens with curative potential for patients with B-cell lymphomas.
Introduction
Given the importance of B-cell leukemia/lymphoma-2 (BCL-2) in the pathophysiology of B-cell lymphomas, these diseases were a natural first choice of cancers to target with BCL-2 inhibition.1 However, effectively targeting this pathway in patients remained elusive for several years, and questions arose as to the validity and feasibility of inhibiting this target. Recently, the BCL-2–specific antagonist venetoclax has proven to be highly effective in chronic lymphocytic leukemia (CLL), leading to an initial approval in this disease. Notable activity has also been observed in other B-cell non-Hodgkin lymphoma (NHL) subtypes. Here, I review the turbulent early attempts at targeting BCL-2, the current success and limitations of venetoclax, and my perspectives on where this field may head in the future.
BCL-2 biology
The BCL-2 family of proteins, with more than a dozen members, is the key regulatory cascade governing the biology of the intrinsic pathway of mitochondrial apoptosis, a physiologic process of programmed cell death.2 BCL-23 itself is an antiapoptotic protein and part of a group of such proteins that also includes MCL-1,4 BCL-XL,5 BCL-w,6 and BFL-1,7 which all promote cell survival. A second group of proapoptotic proteins, known as sensitizer BH3-only proteins, includes BIM, tBID, BAD, PUMA, NOXA, HRK, and others.8-11 These proteins promote cell death by binding to specific antiapoptotic proteins and/or the proapoptotic effectors. This latter group includes BAX12,13 and BAK,14 which when activated homo-oligomerize and lead to mitochondrial outer-membrane permeabilization, a point of no return that leads to cytochrome c release, caspase activation, and subsequent apoptotic cell death.15
As a whole, the BCL-2 family acts as a precise intracellular sensor that regulates the critical decision of whether a cell lives or dies. In normal cells, a balance of anti- and proapoptotic proteins protects cells as they encounter everyday cellular stress, but also systematically induces death in damaged cells. Cancer cells can hijack this system by tipping the balance toward antiapoptotic proteins, thereby facilitating prolonged tumor-cell survival. One particularly attractive aspect of targeting the BCL-2 family therapeutically is that it lies downstream of the tumor suppressor protein TP53, which is frequently dysfunctional in B-cell malignancies, leading to resistance to traditional cytotoxic chemotherapy.16
BCL-2 in B-cell lymphomas
B-cell NHLs were the logical first diseases in which to explore the use of BCL-2 inhibitors. Indeed, BCL-2 itself was first cloned in a lymphoid cell line,17 and a translocation juxtaposing BCL-2 to the immunoglobulin heavy chain promoter t(14;18) is considered to be the key pathophysiologic event in the development of follicular lymphoma (FL).18 Chromosome 18q21 amplification leading to high BCL-2 protein levels is also observed in a subset of patients with mantle cell lymphoma (MCL),19 and BCL-2 overexpression is present in ∼30% of cases of diffuse large B-cell lymphoma (DLBCL).20 The rationale for targeting BCL-2 therapeutically is also strong in CLL, the most common leukemia in the Western world. It was previously shown that 13q deletion [del(13q)], the most common cytogenetic abnormality in CLL, leads to deletion of miR-15 and miR-16, microRNAs that act as negative regulators of BCL-2.21 This deletion leads to high BCL-2 expression in this CLL subtype, with less well-understood mechanisms resulting in nearly uniformly high BCL-2 expression across all CLL subtypes.22,23
Early efforts to target BCL-2
Given the compelling biological rationale for targeting BCL-2 in B-cell lymphomas, a number of agents of questionable specificity and potency were initially explored in the clinic. Oblimersen, an antisense oligodeoxynucleotide designed to specifically target BCL-2 mRNA,24 had been shown to decrease BCL-2 expression and tumor-cell proliferation in B-cell lymphoma cell lines.25 In an early-phase study in CLL, the single-agent efficacy of oblimersen was limited, with only 2 (8%) of 26 evaluable patients having a partial response (PR).26 A later phase 3 trial randomly assigned patients to fludarabine/cyclophosphamide with or without oblimersen, and no significant difference in 5-year overall survival was observed.27
Gossypol compounds, natural polyphenolic aldehyde derivatives isolated from the cotton plant, interact with a variety of antiapoptotic BCL-2 family proteins,28,29 and treatment of primary CLL cells with these compounds led to apoptosis ex vivo.30 One derivative known as AT-101, or apo-gossypol, had limited efficacy in a phase 1 trial in relapsed/refractory CLL and was associated with transaminitis.31 A phase 2 study of AT-101 plus rituximab in relapsed/refractory CLL found a modest overall response rate (ORR) of 44%, with no complete responses (CRs).32
A third approach, obatoclax (formerly GX15-070), was derived from a screen of molecules that disrupted BCL-2 family protein/protein interactions.33 Although obatoclax targets BCL-2 and induces apoptosis in primary CLL cells in vitro, it also binds to the other antiapoptotic proteins MCL-1, BCL-XL, BCL-w, and MCL-1 at micromolar concentrations.34 In the phase 1 experience in CLL, neurologic symptoms such as somnolence and ataxia were found to be dose limiting, and only 1 (4%) of 26 patients achieved response.35 A phase 1 trial of obatoclax in combination with fludarabine and rituximab for relapsed/refractory CLL had a PR rate of 54%,36 which was not clearly better than historical results for this chemoimmunotherapy regimen alone.
Thus, despite several early attempts, finding an effective and well-tolerated drug to selectively target BCL-2 initially proved to be elusive. It was unclear whether this was due mainly to the lack of a drug specific for BCL-2 or whether, because of resistance mechanisms, BCL-2 itself was not as promising a target as had been originally hoped. Fortunately, given the compelling biology of BCL-2 dependence in the pathophysiology of B-cell lymphomas, additional efforts were pursued.
Navitoclax
To develop a more potent and specific inhibitor of BCL-2, a screen was performed for small molecules that block the hydrophobic BH3-binding pocket on BCL-XL.37 This screen identified ABT-737, a molecule that binds to BCL-2, BCL-XL, and BCL-w with high affinity. ABT-737 was found to have promising activity in preclinical studies in lymphoid malignancies such as CLL.23 The orally bioavailable version of ABT-737, navitoclax (formerly ABT-263),38 subsequently moved into clinical trials for patients with B-cell lymphomas.
Fifty-five patients with lymphoid malignancies were treated in a phase 1, first-in-human study of navitoclax monotherapy.39 All 7 patients with CLL had a >50% reduction in lymphocytosis, and 6 of 16 patients with FL had a decrease in lymph node size. However, grade 3/4 thrombocytopenia was observed in 29% of patients, which proved to be a dose-limiting toxicity. This was likely due to the fact that BCL-XL is an important prosurvival factor for platelets,40 and on-target BCL-XL inhibition by navitoclax led to apoptosis in older platelets. Navitoclax was further explored in a dedicated phase 1 study in relapsed/refractory CLL.41 Nine (31%) of 29 patients achieved a PR, with a median progression-free survival (PFS) of 25 months in this heavily pretreated group of patients. More recently, a randomized phase 2 study of rituximab with or without navitoclax in previously untreated CLL was reported, with ORRs of 55% and 35%, respectively.42
Venetoclax in CLL
Despite the promise of navitoclax, there was concern that the thrombocytopenia caused by the drug would limit dose-intensity and thus efficacy. Therefore, a more specific and even more potent BCL-2 inhibitor was developed. Venetoclax (formerly ABT-199/GDC-0199) is an orally bioavailable BCL-2 inhibitor with subnanomolar affinity for BCL-2 (Ki < 0.010 nM) that binds with >3 orders of magnitude less avidity to BCL-XL and BCL-w and has no measurable binding to MCL-1.43 Venetoclax induces apoptosis across a diverse array of hematological cancer-cell lines and primary CLL cells. Moreover, the drug led to substantial tumor regression in 4 mouse xenograft models and did not show any significant dose-dependent thrombocytopenia.
Venetoclax monotherapy in CLL
On the basis of the promising preclinical data, a phase 1 first-in-human study of venetoclax in relapsed/refractory CLL and NHL (M12-175) was launched in 2011. The initial 3 patients all had relapsed/refractory CLL with bulky lymph node disease and/or substantial lymphocytosis.43 At a starting dose of 200 or 100 mg, dramatic reductions in absolute lymphocyte count and lymph node size were noted within 8 hours of initial dosing. Over the same timeframe, laboratory changes such as rapidly rising lactate dehydrogenase consistent with tumor lysis syndrome (TLS) were observed. With careful management, all 3 patients had resolution of TLS without clinical sequelae and were able to safely resume venetoclax dosing and achieve response. The experience of these initial patients demonstrated the power of this new agent and highlighted the need to take precautions to prevent TLS when initiating venetoclax therapy in those with CLL.
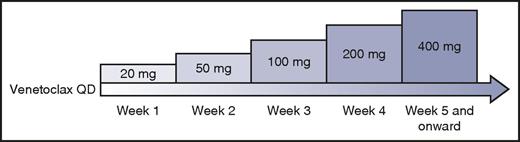
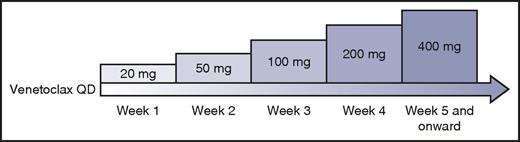
After this initial experience, the study was amended to include a lower starting dose of 50 mg and mandate careful prophylaxis, monitoring, and management of TLS. A weekly intrapatient dose ramp-up scheme was implemented (Figure 1). Using this algorithm, 56 patients with CLL were treated in a dose-escalation cohort, with daily venetoclax administered until disease progression or unacceptable toxicity, with dose levels up to 1200 mg daily explored.44 Despite the previously instituted safety measures, 3 episodes of clinical TLS occurred, including 1 death likely resulting from TLS in a patient treated at 1200 mg.
Intrapatient dose ramp-up scheme for patients with CLL initiating venetoclax. Professional illustration by Patrick Lane, ScEYEnce Studios.
Intrapatient dose ramp-up scheme for patients with CLL initiating venetoclax. Professional illustration by Patrick Lane, ScEYEnce Studios.
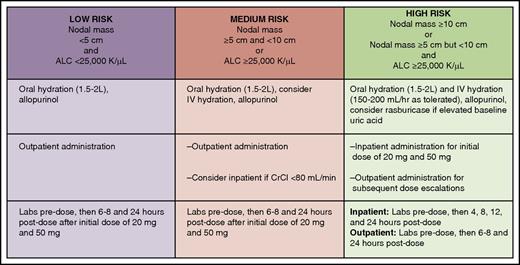
The dose-escalation scheme was further modified to include an even lower starting dose of 20 mg, and more stringent TLS risk stratification, prophylaxis, and monitoring were implemented (Figure 2). After these revisions, an additional 60 patients with CLL were accrued in an expansion cohort treated after ramp-up to a dose of 400 mg daily, and no additional events of clinical TLS occurred. Other common toxicities observed with venetoclax were grade 1/2 gastrointestinal adverse effects such as nausea and diarrhea in about half of patients and grade 3/4 neutropenia in ∼40% of patients. The latter toxicity is likely due to the reliance of neutrophil precursor cells on BCL-2 for survival.45 Despite the significant frequency of neutropenia, the rate of grade 3/4 febrile neutropenia was low at 6%.
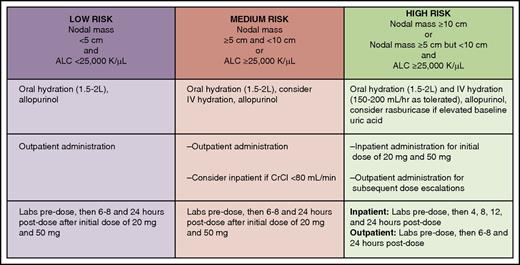
Tumor lysis syndrome risk stratification, prophylaxis, and monitoring for patients with CLL initiating venetoclax. ALC, absolute lymphocyte count; CrCl, creatinine clearance. Professional illustration by Patrick Lane, ScEYEnce Studios.
Tumor lysis syndrome risk stratification, prophylaxis, and monitoring for patients with CLL initiating venetoclax. ALC, absolute lymphocyte count; CrCl, creatinine clearance. Professional illustration by Patrick Lane, ScEYEnce Studios.
Venetoclax was found to be efficacious across dose levels. Among the 116 total patients in the study, the ORR was 79%, with 20% of patients achieving complete remission. Deep responses were observed in all 3 compartments, including peripheral blood, lymph nodes, and bone marrow. Response rates were equivalent even in high-risk patients, including those with del(17p), although this latter group did have a shorter PFS. In a recent update on patients who received the recommended phase 2 dose of 400 mg per day, the estimated 24-month PFS was 62%.46 Biomarkers to predict the quality of response to venetoclax were also evaluated in this trial. Using BH3 profiling, a functional assay that assesses the proximity of a cell to the threshold of apoptosis (mitochondrial priming), we found that patients with highly primed CLL cells at baseline had deeper responses, particularly in the bone marrow.47
To further explore the efficacy of venetoclax in the high-risk, relapsed/refractory del(17p) population, a landmark phase 2 study enrolled 107 patients who were treated with venetoclax with a similar intrapatient dose ramp-up to 400 mg daily.48 The ORR of 79% was identical to that observed in the phase 1 study, and with a relatively short follow-up of 12 months, CR was noted in 8% of patients. On the basis primarily of data from these phase 1 and 2 studies, venetoclax received accelerated US Food and Drug Administration approval in April 2016 for the treatment of patients with del(17p) CLL who have relapsed after or are refractory to ≥1 prior line of therapy. It has subsequently received similar approvals in Europe and several other countries around the world.
Another specific area of unmet medical need in CLL is progression during treatment with B-cell receptor pathway inhibitors. Given its distinct mechanism of action, venetoclax was a logical agent to explore in this population. An ongoing phase 2 trial is evaluating venetoclax in patients with CLL who have experienced progression with ibrutinib or idelalisib. It should be noted that ∼40% of the patients experienced progression after stopping their kinase inhibitor because of intolerance, a less high-risk group than those who experience progression while receiving kinase inhibitors. For evaluable patients who had experienced progression with ibrutinib (n = 43) or idelalisib (n = 21), the ORR was promising at 70% and 48%, respectively.49 Few CRs were observed, although follow-up at this time remains short, and in prior venetoclax studies, some responders did not achieve a CR until they had been receiving the drug for at least 1 year.
Several other important practical considerations with venetoclax administration in CLL are worth mentioning. Given that nearly half of patients will experience grade 3/4 neutropenia, it is important to note that growth factor support such as pegfilgrastim can be provided concomitantly with venetoclax and should typically be considered when the absolute neutrophil count drops below 1000/uL. Dose interruption or dose reduction should be reserved for persistent neutropenia despite growth factor administration. Because food affects the peak serum concentration and area under the concentration-time curve of the drug, venetoclax should be administered with food, although no specific fat content is needed.50 The drug is metabolized by CYP3A, and therefore, strong CYP3A inducers should be avoided, with the venetoclax dose decreased when strong CYP3A inhibitors need to be administered concomitantly. Because of the narrow therapeutic window of warfarin, it is preferable to use alternative anticoagulants in patients receiving venetoclax.51
Venetoclax combination therapy in CLL
Although BCL-2 inhibition alone is effective in CLL, most patients do not achieve a CR, and response durability for most patients is suboptimal. Thus, exploring combination strategies with venetoclax was the logical next step in the development of the drug. Preclinical data suggested that adding the anti-CD20 antibody rituximab to venetoclax might enhance antibody-dependent cytotoxicity.43
In a phase 1b study of venetoclax plus rituximab in 49 patients with relapsed/refractory CLL, the toxicity profile of the combination was similar to that of venetoclax alone, perhaps with somewhat higher rates of neutropenia (grade 3/4, 53%).52 Early in the study, 1 death resulting from TLS was observed after an initial 50-mg dose in a patient with bulky lymphadenopathy treated before study amendments that introduced additional safety measures to mitigate TLS risk. The venetoclax plus rituximab combination was highly active, with an ORR of 86%, CR/CR with incomplete recovery of blood count rate of 51%, and a 2-year PFS estimate of 82%. Fifty-seven percent of patients achieved minimal residual disease (MRD) negativity by flow cytometry (sensitivity, 10−4) in marrow. Thirteen responders elected to cease therapy, and all 11 who were MRD negative at time of cessation remained progression free at the time the study was published. Although patients in this study were less heavily pretreated than those in the phase 1 study (median of 2 vs 3 prior therapies, respectively), the high rate of CRs and MRD negativity and the prospect of time-limited therapy with this regimen are promising. A randomized, phase 3 registration study of a 2-year course of venetoclax plus rituximab vs bendamustine plus rituximab in relapsed/refractory CLL is now fully accrued (NCT02005471) and, if positive, would likely lead to full approval of venetoclax plus rituximab in the United States and elsewhere.
The combination of venetoclax with the type 2 glycoengineered anti-CD20 antibody obinutuzumab is currently being evaluated in CLL14, a randomized, phase 3 trial comparing 1 year of obinutuzumab/venetoclax with obinutuzumab/chlorambucil in previously untreated, older patients with CLL. A preliminary analysis of 13 patients in the safety run-in phase found the combination to be well tolerated and have promising efficacy, with 7 of 12 patients achieving a CR/CR with incomplete recovery of blood count and 11 of 12 achieving MRD negativity in the blood.53
In addition to anti-CD20 antibodies, venetoclax combinations with chemotherapy and other novel agents are also being pursued in CLL, as summarized in Table 1. Ongoing studies include venetoclax in combination with bendamustine plus rituximab or bendamustine plus obinutuzumab (NCT01671904) and sequential bendamustine debulking followed by venetoclax with obinutuzumab induction and maintenance in CLL (NCT02401503).54
One particularly promising combination partner for venetoclax is the Bruton tyrosine kinase (BTK) inhibitor ibrutinib. This 2-drug combination leads to potent CLL cell killing ex vivo.55 Moreover, using BH3 profiling, we recently found that venetoclax and ibrutinib have distinct and complementary effects on CLL-cell mitochondria.56 Specifically, venetoclax increases mitochondrial priming in CLL cells, whereas ibrutinib leads to CLL cells becoming selectively more BCL-2 dependent. Several clinical trials involving the venetoclax plus ibrutinib combination are under way, including a phase 2 study in previously untreated CLL (NCT02756897) and 2 different phase 2 studies with a 3-drug combination of venetoclax, ibrutinib, and obinutuzumab in previously untreated patients with del(17p) CLL (NCT02758665) or in patients with CLL irrespective of cytogenetic risk group (NCT02427451).
Venetoclax in NHL
In parallel with the CLL arm of the phase 1 first-in-human study of venetoclax, a second arm accrued 106 patients with NHL, including DLBCL (n = 34), FL (n = 29), MCL (n = 28), Richter transformation (n = 7), Waldenström macroglobulinemia (n = 4), and marginal zone lymphoma (n = 3).57 Both the toxicity and efficacy profiles were in general more modest than those observed in CLL. For example, grade 3/4 neutropenia was only observed in 11% of patients, and no clinical TLS occurred. The ORR for the entire cohort was 44%. Venetoclax was most active in MCL, where a 75% ORR rate and 21% CR rate were observed, with a median PFS of 14 months. Despite the BCL-2 translocation known to underlie the biology of FL, the ORR in FL was 38%, with a median PFS of 11 months. It is unclear at this time why the activity of venetoclax in FL is less than in CLL. One hypothesis is that other antiapoptotic proteins may provide a more protective milieu for lymphoma cells in the lymph node microenvironment. A slightly higher ORR of 44% was noted in patients with FL treated at ≥1200 mg compared with 27% in those treated at ≤900 mg, suggesting that higher dosing could be explored as one potential strategy to augment efficacy in lymph nodes and thereby improve response rates in FL. Only 18% of patients with DLBCL responded, with no clear association between BCL-2 protein expression level and response.
As in CLL, combination regimens are currently under evaluation in NHL (Table 1). A phase 2 study of venetoclax plus rituximab vs venetoclax plus bendamustine/rituximab is ongoing in patients with relapsed/refractory FL (NCT02187861). In a preliminary analysis, an ORR with venetoclax plus rituximab of 33% was observed in a highly refractory population, and a 64% ORR was observed with the venetoclax plus bendamustine/rituximab combination.58 A combination of venetoclax with obinutuzumab in previously untreated FL is also currently under way (NCT02877550). As in CLL, the combination of ibrutinib and venetoclax is being evaluated in a phase 2 study in relapsed/refractory MCL (NCT02471391). The early experience in this trial suggests good tolerability and promising efficacy of the combination.59 There are also ongoing studies exploring whether venetoclax may act as a chemosensitizing agent, such as a study of venetoclax administered in combination with R-CHOP or obinutuzumab-CHOP in previously untreated DLBCL (NCT02055820). A preliminary report found that, as expected, CR rates were high, but follow-up was too short to assess PFS. The toxicity profile was acceptable, but venetoclax dosing had to be reduced to administration only on days 1 to 10 (rather than continuous dosing) to mitigate the rate of grade 3/4 neuropenia.60 We recently launched a phase 2 study of venetoclax in combination with dose-adjusted R-EPOCH (rituximab plus etoposide, prednisone, vincristine, cyclophosphamide, and doxorubicin) chemotherapy in patients with CLL with Richter transformation to DLBCL (NCT03054896). A study with a similar regimen is also under way for patients with de novo aggressive B-cell lymphomas such as double-hit DLBCL (NCT03036904).
Opposing viewpoints
Given the complexity of the BCL-2 family of proteins, a reasonable observer of this field may fairly wonder why selectively targeting BCL-2 alone would be effective in treating B-cell lymphomas. In CLL, where response rates to venetoclax monotherapy are high across all different risk groups, the durability of response has been variable. In NHL, responses have been less consistent and raise further doubt that venetoclax monotherapy will provide durable benefit for most patients. A potential resistance mechanism in tumor cells faced with selective BCL-2 inhibition would be a shift to dependency on alternative antiapoptotic proteins such as MCL-1 or BCL-XL. Another possible mechanism would be selection for BCL-2 mutations that confer direct resistance to venetoclax. Preclinical models have already suggested the plausibility of such resistance mechanisms,61,62 although these mechanisms have not yet been convincingly demonstrated in samples from patients experiencing progression with venetoclax. Nonetheless, a fair argument can be made that venetoclax monotherapy is not likely to provide durable clinical benefit for many patients with B-cell lymphomas.
Additional investigations
The inherent limitations of targeted agents used as monotherapy to treat B-cell lymphomas are not unique to venetoclax. For example, BTK resistance mutations have already been clearly documented in patients with CLL receiving ibrutinib.63 Given the experience with cancer chemotherapy in the last century, perhaps this should not be a surprise. In the era of cytotoxic chemotherapy, we learned that, compared with sequential single-agent use, combining efficacious agents with nonoverlapping toxicities leads to more durable remissions and in some cases cure. Given the number of available agents and the rising cost of clinical trials, carefully designed preclinical studies to evaluate novel drug combinations relevant to B-cell lymphomas in robust model systems will be essential. In addition to work done directly with primary patient samples, additional models such as dynamic BH3 profiling,64 novel cell lines,65 mouse models,66 and patient-derived xenografts67 will all be useful to generate data to inform the design of future combination clinical trials.
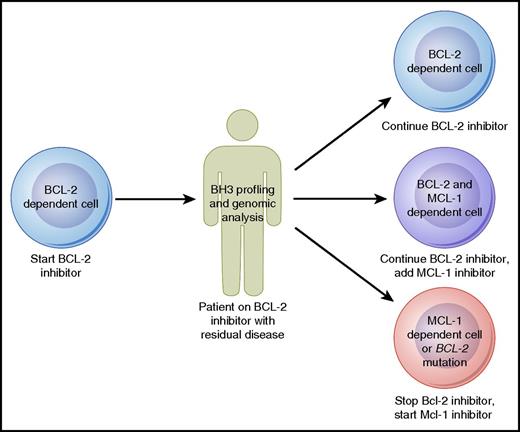
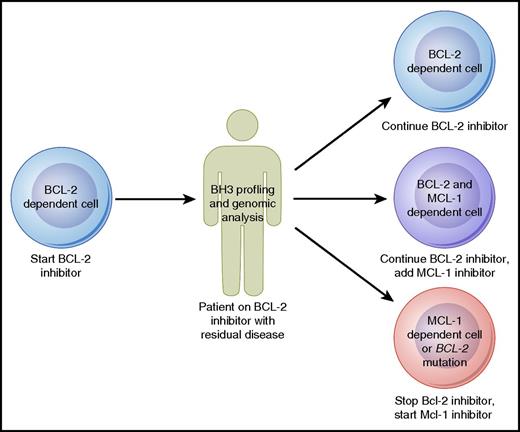
Strategies to proactively monitor patients for early signs of drug resistance may also prove to be beneficial. Such a strategy has already begun to be employed in patients receiving ibrutinib, who can be tested serially for the development of BTK resistance mutations that may prompt consideration of a change in therapy before actual clinical progression occurs.68 Although such genomic evaluations are not yet available for patients treated with venetoclax, they may prove to be useful in the future to monitor patients for BCL-2 mutations. A complementary approach to genomic evaluation is the BH3 profiling assay, which in addition to assessing priming for apoptosis can also functionally evaluate the antiapoptotic dependence of malignant cells. Baseline mitochondrial priming has already shown promise in predicting depth of response to venetoclax in CLL.47 Serial tumor sampling from patients receiving venetoclax could allow for BH3 profiling to be used for ongoing monitoring. For example, a patient responding well to venetoclax monotherapy might be found initially to have cells dependent primarily on BCL-2 for survival, but over time, these cells may shift dependency to rely more on MCL-1. Given the steady progress in the development of MCL-1–selective inhibitors,69 one could imagine that eventually this information could be used to make a decision about whether to add on or switch to an MCL-1 inhibitor (Figure 3). As these genomic and functional biomarkers mature, they will need to be studied and validated in prospective clinical trials before being used to make clinical decisions.
Model for how BH3 profiling and genomic analysis could help guide therapy with agents targeting the BCL-2 family of proteins in B-cell lymphomas. Professional illustration by Patrick Lane, ScEYEnce Studios.
Model for how BH3 profiling and genomic analysis could help guide therapy with agents targeting the BCL-2 family of proteins in B-cell lymphomas. Professional illustration by Patrick Lane, ScEYEnce Studios.
Conclusions
The recent approval of venetoclax has demonstrated that BCL-2 inhibition is an important new approach in treating B-cell lymphomas. Venetoclax is highly active even as monotherapy in CLL and MCL, with activity also observed in several other NHL subtypes. Combination strategies with a diverse array of other agents are now being explored and will likely have a major impact on how we treat B-cell lymphomas. Genomic and functional preclinical studies will be crucial to determine which novel-agent combinations are worthy of study in the clinic, how resistance arises, and how to overcome it.
A previous generation of oncologists developed powerful chemotherapeutic agents and devised time-limited combination regimens with curative potential for aggressive lymphomas. With the advent of venetoclax and several other new active, targeted therapies in B-cell lymphomas, we now have a similar opportunity to devise novel agent–only combination regimens with less toxicity than chemotherapy and the potential for even greater efficacy. Although some older patients with comorbidities may be appropriate for indefinite novel-agent monotherapy, we should remain ambitious in how we approach the treatment of younger, fit patients. Given the significant activity of venetoclax and other novel agents, our goal for such fit patients, even those with traditionally incurable indolent diseases such as CLL, should be to develop time-limited novel-agent combination regimens with good tolerability and curative potential. Such studies, including several combining venetoclax with ibrutinib, are already under way and hold tremendous promise for the treatment of patients with B-cell lymphomas in the future.
Acknowledgments
M.S.D. has a Career Development Award from the American Society of Clinical Oncology.
Authorship
Contribution: M.S.D. wrote the manuscript.
Conflict-of-interest disclosure: M.S.D. has consulted for AbbVie, Genentech, Pharmacyclics, Janssen, TG Therapeutics, Gilead, Infinity, Celgene, Merck, and Incyte.
Correspondence: Matthew S. Davids, Department of Medical Oncology, Dana-Farber Cancer Institute, 450 Brookline Ave, Boston, MA 02215; e-mail: matthew_davids@dfci.harvard.edu.