Abstract
Obinutuzumab (OBZ) is a recombinant type II anti-CD20 and immunoglobulin G1 Fc-optimized monoclonal antibody (mAb), recently approved in chronic lymphocytic leukemia (CLL; B-cell CLL) and follicular lymphoma (FL). Rituximab (RTX) is frequently considered as its “ancestor” and OBZ clinical development was justified by the importance of FcγRIIIA-mediated mechanisms in RTX clinical activity. However, RTX differs from OBZ in 2 critical independent properties: being a type I anti-CD20 mAb and not being Fc-optimized. Moreover, the use of a different dosing regimen for RTX and OBZ further complicates any interpretation of clinical results. The results obtained for OBZ in CLL provide new arguments for FcγRIIIA-mediated mechanisms when the target antigen is expressed at a low density. Results of OBZ in FL confirm the interest for FcγRIIIA-mediated mechanisms, with some limitations, some of them being possibly due to lack of OBZ-induced complement activation. The situation in diffuse large B-cell lymphoma is deceiving, as the possible gains of activity of OBZ appear to be annihilated by the lack of complement activation. Although RTX was by chance an anti-CD20 mAb with equilibrated pharmacodynamic properties, the reinforcement of some of these properties, which has been done at the expense of complement activation, has conferred an advantage in some B-cell disorders while restricting OBZ indications. The OBZ story nicely demonstrates that the future of naked mAbs is to design agents with optimized and tailored properties, and that this must be done step by step, with a full clinical validation.
Introduction
Obinutuzumab (OBZ) is the first recombinant type II anti-CD20 and immunoglobulin G1 (IgG1) Fc-optimized monoclonal antibody (mAb) approved by the US Food and Drug Administration (FDA) and European Medicines Agency. It is currently labeled in first-line chronic lymphocytic leukemia (CLL) patients in association with chlorambucil, and in combination with bendamustine followed by OBZ monotherapy for the treatment of patients with follicular lymphoma (FL) who relapsed after, or are refractory to, a rituximab (RTX)-containing regimen. RTX was the first anti-CD20 mAb approved and largely contributed to establish the therapeutic value of anti-CD20 strategies in almost all CD20+ malignancies. Experimental studies demonstrated that RTX is able to induce CD20+ cell death by several mechanisms.1 RTX is sometimes presented as the “ancestor” of OBZ. First of all, OBZ is in no way a RTX biosimilar: not only are their variable domains totally different but also, in addition, RTX does not recognize the same CD20 epitope as OBZ and is a human IgG1 non-Fc-optimized antibody, 2 characteristics independent from each other and having functional and pharmacological consequences that need to be taken into consideration when trying to compare the 2 drugs.
OBZ development was largely based on the presumed importance of FcγRIIIA-mediated mechanisms in RTX clinical activity, this being mainly supported by the influence of the FcγRIIIA-158VF polymorphism in clinical response found in some FL studies.2,3 Inspired by our initial discovery, GlycArt Biotechnology first applied their glycoengineering GlycoMAb technology to optimize RTX in its Fc portion to improve mAb-FcγRIIIA interaction4,5 and then decided to develop their own Fc-optimized anti-CD20 antibody, called GA101 or OBZ.6 To clearly depart from RTX, the GA101 parental murine anti-CD20 antibody was chosen to have unusual characteristics, that is, the ability to induce homotypic adhesion, a typical property of type II anti-CD20 mAbs. This property could be linked to a different manner to recognize the CD20 tetramers, as compared with RTX and other type I anti-CD20 mAbs. As a consequence, OBZ does not exert complement-dependent cytotoxicity (CDC) and displays a different mechanism of direct cytotoxicity compared with RTX.6,7
A prospective randomized phase 3 study demonstrated the superiority of immunochemotherapy associating OBZ and chlorambucil compared with RTX-chlorambucil in first-line CLL patients.8 Improved progression-free survival (PFS) was recently shown in first-line FL9 and in relapsed/refractory (R/R) FL.10,11 If the clinical advantage of OBZ over RTX is taking shape, no advantage was demonstrated in diffuse large B-cell lymphoma (DLBCL).12 These divergent results remain currently misunderstood and led to questioning whether experimental advantages over RTX differentially translate in humans according to histology. These clinical differences could point to certain mechanisms of action of anti-CD20 mAb therapy and/or to the immune context, according to histology.
OBZ: a unique combination of pharmacodynamic properties
Fab-linked properties
OBZ was obtained by grafting the complementarity-determining region sequences from the murine antibody B-ly1 into human IGHV1-69*08 and IGHJ5*01-encoded acceptor frameworks for the VH and human IGKV2D-29*02 and IGKJ4*01 (or IGKJ4*02) for the VL.6,13 OBZ is a type II anti-CD20 mAb and thus differs from most recombinant anti-CD20 mAbs, which belong to the type I (RTX, ofatumumab, ocaratuzumab, ublituximab) (Figure 1). The seminal difference is their ability to induce CDC (type I) or not (type II),14 a characteristic that is related to their ability (type I) or inability (type II) to induce mAb-CD20 complex translocation into lipid rafts (Table 1), which gathers Fc portions and favors hexamer formation and C1q recruitment.15,16 Only type I mAbs induce a significant level of CDC at low concentrations and low antigen density. At high concentrations, at least in vitro, OBZ induces similar levels of CDC as RTX and ofatumumab.17 Other characteristics of type II anti-CD20 mAbs differentiate them from type I: homotypic adhesion resulting in caspase-independent direct cell death, half-maximal CD20 binding at saturating conditions, less or no CD20 modulation. These type II–specific in vitro properties are described for OBZ,6,17,18 and translate into an enhanced direct cell cytotoxicity compared with RTX.19 These unique properties of OBZ probably rely on the architecture of immune complexes formed at the surface of CD20+ cells. OBZ and RTX bind to highly overlapping epitopes of CD20, but in a totally different orientation (Figure 2).7,20 In comparison with RTX, OBZ is rotated 90° around the RTX Fab middle axis and tilts 70° toward the C terminus of the CD20 epitope. Moreover, the elbow angle between VH and CH1 is 30° wider and involves the residue at position 13 in the VH (Figure 2), which was shown to be critical in the activity of OBZ.6 A full understanding of the difference between OBZ and RTX would require knowing the precise structure of membrane CD20 and the spatial arrangement of the tetramers at the membrane surface, which is not yet the case. Taking into account the half-maximal CD20 binding at saturating conditions, OBZ is thought to engage its 2 Fab arms on the same CD20 tetramer, like crab claws, whereas RTX, like other type I anti-CD20 mAbs, would be able to bridge only 2 CD20 tetramers.20 By modifying the hinge region of RTX (Figure 2), it was possible to dramatically increase its proapoptotic potential,21 suggesting that the proapoptotic activity of type I anti-CD20 mAbs could be reinforced by playing with their ability to cross-link CD20 tetramers.
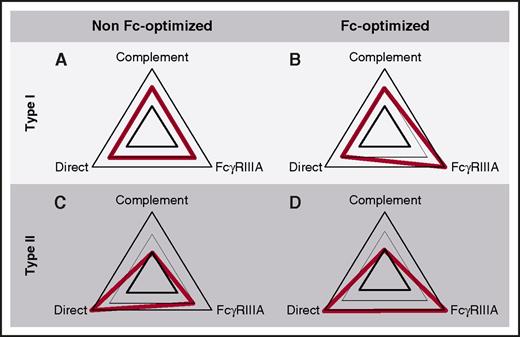
Pharmacodynamic profile of anti-CD20 mAbs. (A) Type I, non-Fc-optimized mAbs. As a typical type I anti-CD20 IgG1 mAb, RTX is able to eliminate target cells through complement activation, recruitment of FcγRIIIA-expressing effector cells, and also to some extent by direct cytotoxicity. (B) Type I, Fc-optimized mAbs. This category of mAbs includes ocaratuzumab and ublituximab. They behave similarly as in panel A, except for FcγRIIIA-dependent mechanisms, which are exhausted. (C) Type II, non-Fc-optimized mAbs. This is currently a virtual category because no mAb of this type is currently under development. As compared with panel A, they would have an enhanced direct cytotoxicity effect, and a reduced ability to trigger complement activation. (D) Type II, Fc-optimized mAbs, such as OBZ. These mAbs have a reduced ability to activate complement, but an enhanced ability to recruit FcγRIIIA-expressing cytotoxic and phagocytic effectors and to induce direct cytotoxicity.
Pharmacodynamic profile of anti-CD20 mAbs. (A) Type I, non-Fc-optimized mAbs. As a typical type I anti-CD20 IgG1 mAb, RTX is able to eliminate target cells through complement activation, recruitment of FcγRIIIA-expressing effector cells, and also to some extent by direct cytotoxicity. (B) Type I, Fc-optimized mAbs. This category of mAbs includes ocaratuzumab and ublituximab. They behave similarly as in panel A, except for FcγRIIIA-dependent mechanisms, which are exhausted. (C) Type II, non-Fc-optimized mAbs. This is currently a virtual category because no mAb of this type is currently under development. As compared with panel A, they would have an enhanced direct cytotoxicity effect, and a reduced ability to trigger complement activation. (D) Type II, Fc-optimized mAbs, such as OBZ. These mAbs have a reduced ability to activate complement, but an enhanced ability to recruit FcγRIIIA-expressing cytotoxic and phagocytic effectors and to induce direct cytotoxicity.
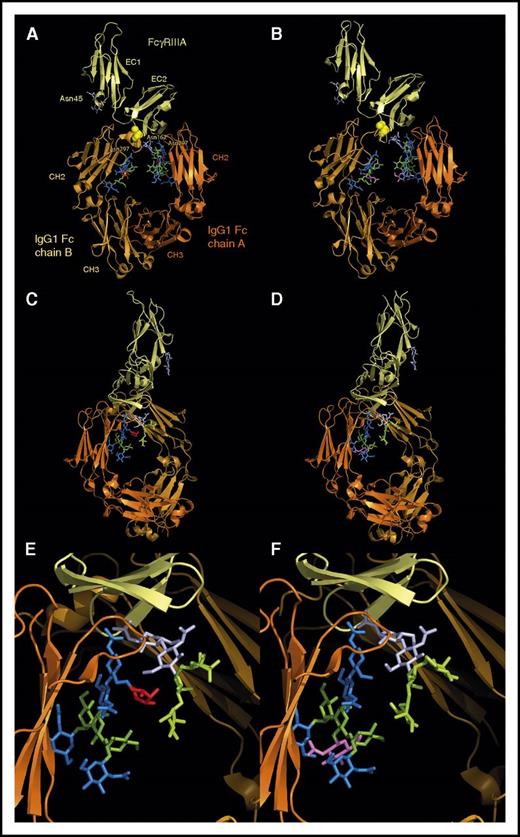
Mechanics of CD20 recognition by anti-CD20 antibodies, and its relationship with direct cytotoxicity. (A) RTX and OBZ bind roughly the same epitope on the larger loop of CD20 but, depending on the recognition of Asn76 (OBZ and type II mAbs) or not (RTX and type I mAbs),7 the paratope shifts by a few notches, modifying the orientation of the Fab arm (blue arrow) relative to CD20 and the target cell membrane. (B) During the process of humanization of the parental murine mAb of GA101 (OBZ), the white-circled purple residue at position 13 (IMGT unique numbering) was demonstrated to be critical direct cytotoxicity, and must be a valine.6 This residue is very close to Phe150 (Eu numbering) in the CH1 domain, and is thought to be responsible for the wider elbow angle of OBZ relative to RTX.7 Tightening or loosening the screw responsible for this elbow hinge is therefore another way to influence direct cytotoxicity. (C) Replacing the serine residues 131 (CH1) or 229 (hinge) (Eu numbering) of RTX by cysteines, as in human IgG2, enhances RTX-mediated apoptosis and tends to create conditions of half maximal binding to CD20, as for type II mAbs.21 These residues probably create different inter–heavy chain disulfide bridges, rendering the hinge more rigid and also widening the Fab-Fab angle. Here again, tightening or loosening the screw of this hinge is another way to influence direct cytotoxicity.
Mechanics of CD20 recognition by anti-CD20 antibodies, and its relationship with direct cytotoxicity. (A) RTX and OBZ bind roughly the same epitope on the larger loop of CD20 but, depending on the recognition of Asn76 (OBZ and type II mAbs) or not (RTX and type I mAbs),7 the paratope shifts by a few notches, modifying the orientation of the Fab arm (blue arrow) relative to CD20 and the target cell membrane. (B) During the process of humanization of the parental murine mAb of GA101 (OBZ), the white-circled purple residue at position 13 (IMGT unique numbering) was demonstrated to be critical direct cytotoxicity, and must be a valine.6 This residue is very close to Phe150 (Eu numbering) in the CH1 domain, and is thought to be responsible for the wider elbow angle of OBZ relative to RTX.7 Tightening or loosening the screw responsible for this elbow hinge is therefore another way to influence direct cytotoxicity. (C) Replacing the serine residues 131 (CH1) or 229 (hinge) (Eu numbering) of RTX by cysteines, as in human IgG2, enhances RTX-mediated apoptosis and tends to create conditions of half maximal binding to CD20, as for type II mAbs.21 These residues probably create different inter–heavy chain disulfide bridges, rendering the hinge more rigid and also widening the Fab-Fab angle. Here again, tightening or loosening the screw of this hinge is another way to influence direct cytotoxicity.
Fc-linked properties
OBZ has been built as a classical human IgG1κ antibody, but produced in Chinese hamster ovary cells engineered to constitutively overexpress recombinant β-1,4-N-acetyl-glucosaminyltransferase III and Golgi α-mannosidase II, so that the N-glycans attached at asparagine 297 (Eu numbering) in the CH2 domain of the heavy chains are different from that of RTX (Figure 3). Namely, OBZ N-glycans are mostly non–core-fucosylated, facilitating the association with the FcγRIIIA Asn162-linked N-glycan (Figure 3).6 In other terms, OBZ is an engineered IgG1 variant, called G1e5 following the nomenclature we recently proposed,22 and has functional and pharmacological properties distinct from standard IgG1. As compared with RTX, the Fc portion of OBZ demonstrates a higher binding to FcγRIIIA whatever its allotype (−158V or −158F),6 and to FcγRIIIB,23 but binds similarly to FcγRIIA23 and should do also to FcγRI, as afucosylated ocrelizumab does.24 OBZ can thus better recruit natural killer (NK) cells and macrophage through FcγRIIIA to trigger antibody-dependent cell-mediated cytotoxicity (ADCC)6 and neutrophils through FcγRIIIB (and FcγRIIA) to induce antibody-dependent cell phagocytosis (ADCP).23 The use of a G1e5 IgG1 variant also appears to more efficiently stimulate NK cells via the activatory receptor FcγRIIIA, being able to counterbalance competition with complement proteins25 and ADCC inhibition provoked by the interactions between killer inhibitory receptors and major histocompatibility complex class I molecules.26
Structure of a fucosylated and an afucosylated IgG1 Fc bound to FcγRIIIA-158V, as determined by Ferrara et al.85 Top panel, “front” view. The 2 CH2 domains asymetrically bind to the extracellular domain 1 (EC1) of FcγRIIIA. The polymorphic residue FcγRIIIA-158 (represented as spheres) is in close contact with residues of the IgG1 Fc B chain and directly influences Fc-FcγRIIIA interaction. The CH2 N-glycans, anchored at Asn297 (Eu numbering), are face to face in the center of the Fc, and their nonreducing ends point in the direction of the CH3 domains. In the fucosylated Fc (A), the N-glycans (fx1) are composed of 4 GlcNAc residues (blue), 3 Man residues (green), and 1 fucose residue (red), attached to Asn. In the afucosylated Fc (B), the N-glycans (fx2) lack fucose and contain an additional bisecting GlcNAc residue, highlighted in violet. Two FcγRIIIA N-glycans are present: the first one is anchored at Asn45 in the EC2 domain (only its 2 first GlcNAc residues are visible but its antennae should point in the direction of the CH2 domain of the Fc B chain), and the second one at Asn162 (represented with its 2 GlcNAc and its 3 Man residues; fx3) is in great part masked by the N-glycan of the Fc A chain. Whether the Fc is fucosylated (A) or not (B), the overall structure is about the same. Middle panel, “side” view, after a 90° rotation of the top panel. The N-glycan of the Fc B chain has been masked for clarity. The FcγRIIIA Asn162 N-glycan is more visible (paler colors according to the Fc Asn297 N-glycan); its nonreducing ends (high mannose in case of NK cells) diverge from the Fc. These 2 glycans nevertheless directly interact through their core residues (GlcNAc and Fuc in panel C, GlcNAc only in panel D). Bottom panel, Enlargement of the middle panel. The presence of the bisecting GlcNAc residue (violet) in the afucosylated Fc (F) does not modify the orientation of the Fc N-glycan, but the presence of a fucose (E) pushes away the FcγRIIIA Asn162 N-glycan, creating a steric hindrance and reducing Fc-FcγRIIIA affinity. The figures were generated from structures available in the Protein Data Bank, under accession numbers 3SGF for the fucosylated Fc bound to FcγRIIIA-158V and 3SGK for the afucosylated Fc bound to FcγRIIIA-158V, using the PyMOL Molecular Graphics System, version 1.7.4.
Structure of a fucosylated and an afucosylated IgG1 Fc bound to FcγRIIIA-158V, as determined by Ferrara et al.85 Top panel, “front” view. The 2 CH2 domains asymetrically bind to the extracellular domain 1 (EC1) of FcγRIIIA. The polymorphic residue FcγRIIIA-158 (represented as spheres) is in close contact with residues of the IgG1 Fc B chain and directly influences Fc-FcγRIIIA interaction. The CH2 N-glycans, anchored at Asn297 (Eu numbering), are face to face in the center of the Fc, and their nonreducing ends point in the direction of the CH3 domains. In the fucosylated Fc (A), the N-glycans (fx1) are composed of 4 GlcNAc residues (blue), 3 Man residues (green), and 1 fucose residue (red), attached to Asn. In the afucosylated Fc (B), the N-glycans (fx2) lack fucose and contain an additional bisecting GlcNAc residue, highlighted in violet. Two FcγRIIIA N-glycans are present: the first one is anchored at Asn45 in the EC2 domain (only its 2 first GlcNAc residues are visible but its antennae should point in the direction of the CH2 domain of the Fc B chain), and the second one at Asn162 (represented with its 2 GlcNAc and its 3 Man residues; fx3) is in great part masked by the N-glycan of the Fc A chain. Whether the Fc is fucosylated (A) or not (B), the overall structure is about the same. Middle panel, “side” view, after a 90° rotation of the top panel. The N-glycan of the Fc B chain has been masked for clarity. The FcγRIIIA Asn162 N-glycan is more visible (paler colors according to the Fc Asn297 N-glycan); its nonreducing ends (high mannose in case of NK cells) diverge from the Fc. These 2 glycans nevertheless directly interact through their core residues (GlcNAc and Fuc in panel C, GlcNAc only in panel D). Bottom panel, Enlargement of the middle panel. The presence of the bisecting GlcNAc residue (violet) in the afucosylated Fc (F) does not modify the orientation of the Fc N-glycan, but the presence of a fucose (E) pushes away the FcγRIIIA Asn162 N-glycan, creating a steric hindrance and reducing Fc-FcγRIIIA affinity. The figures were generated from structures available in the Protein Data Bank, under accession numbers 3SGF for the fucosylated Fc bound to FcγRIIIA-158V and 3SGK for the afucosylated Fc bound to FcγRIIIA-158V, using the PyMOL Molecular Graphics System, version 1.7.4.
The Fc portion of OBZ should be able to bind C1q, as afucosylated RTX does,27 despite the fact that its C-terminal lysines are not necessarily cleaved, a feature known to modulate IgG hexamerization and hence efficient C1q binding.28 C1q binding and generation of C3b and a membrane attack complex that kills target cells by disrupting cell membrane (CDC) are only obtained at high concentrations of OBZ, mainly because it does not induce OBZ-CD20 translocation in the lipid rafts.17
Concerning their ability to bind FcRn, the Fc receptor responsible for IgG biodistribution and protection against catabolism, there is no obvious reason that OBZ and RTX behave differently, as both are built on an IgG1 backbone and because the CH2 N-glycan core-fucosylation is not known to influence FcRn binding and pharmacokinetics.24,27 Moreover, they share the same G1m (17,1) allotype that was recently shown to influence FcRn binding, and possibly pharmacokinetics.29 It has recently been shown that the variable domains could also influence FcRn-mediated functions,30 a possibility that has never been tested for RTX and OBZ.
What has been learned from preclinical development of OBZ?
The various interspecies differences in IgG and FcγR nature, expression, and function make difficult any definitive interpretation on the impact of Fc modification in animal models.31 In xenograft models, OBZ alone demonstrated superiority to RTX in controlling disease progression.6,32 Moreover, OBZ was able to control tumor progression in RTX-pretreated lymphoma models, suggesting an interest in RTX R/R diseases.17 The combination of chemotherapy and OBZ was demonstrated to be more effective than OBZ monotherapy or RTX-chemotherapy combination.32,33 Cynomolgus monkey model studies demonstrated that both OBZ and RTX induced similar peripheral blood B-cell depletion whereas OBZ induced deeper lymphoid and splenic B-cell depletion.6 In whole blood from healthy donors6,17 and CLL patients,34 OBZ also demonstrated a greater level of B-cell depletion compared with RTX and ofatumumab.
Pharmacokinetics and pharmacodynamics
What has been learned about RTX pharmacokinetics?
It is generally admitted that the dose and the schedule of RTX administration is largely empiric, based on the lack of dose-limiting toxicities and the dose-response relationship observed in phase 1,35,36 as well as the limited size of the good manufacturing processes batch for the first phase 2.37 Nevertheless, the RTX success thereafter demonstrates that the chosen dose was, at least clinically, relevant for most patients.38 PK data and factors affecting RTX exposure and response were well described in a pivotal study,39 showing that exposure to RTX correlates with clinical response, and that antigenic mass and histology affect exposure to RTX. We further demonstrated that FcγRIIIA-158VF polymorphism influenced the rituximab pharmacokinetics/pharmacodynamics relationship, homozygous VV NK cells needing 4 times lower RTX concentration compared with homozygous FF NK cells to obtain a comparable in vitro effect,40 suggesting that certain subsets of patients may benefit from increased dosing. Further studies demonstrated that PK-guided RTX dosing to maintain sufficient RTX concentrations in indolent non-Hodgkin lymphomas (iNHLs),41 or an increased number of RTX infusions in DLBCL,42 failed to demonstrate any clinical advantage. As suggested in a murine model,43 antigen burden is probably, at least in DLBCL, the main parameter affecting RTX exposure. A recent study confirmed that antigenic mass assessed by total metabolic volume before treatment influenced exposure to RTX.44 The theoretical dose of RTX according to tumor burden could be calculated, demonstrating that the optimal dose was close to the approved dose, probably explaining the lack of significant difference in a dose-dense trial.42 The correlation of total metabolic volume before treatment with distribution volumes (VD) without modification of clearance could suggest a “sponge effect,” tumor cells trapping RTX before release of the antibody after exerting its cytotoxic effect.44 In CLL patients, the standard dose of RTX is higher than in non-Hodgkin lymphoma (NHL) patients (500 mg/m2 instead 375 mg/m2), justified by a faster RTX clearance observed in the first phase 2 trial,37,39 leading to low RTX exposure and a worse outcome.45 A benefit for higher RTX doses was demonstrated in some studies,46,47 but a randomized phase 2 study failed to demonstrate any clinical advantage of increased RTX doses (until 6500 mg within 15 days).48 In CLL, the inverse correlation between antigenic mass and RTX clearance suggests a consumption of the antibody by the tumor (“sink effect”), which could not be overcome by an increase the dosing regimen.45,48,49
How do OBZ pharmacokinetics differ from that of RTX?
OBZ clinical development attempted to answer unresolved questions raised by RTX development. Unlike RTX, a fixed dose has been tested in OBZ phase 1.50 This is certainly more convenient in clinical practice, but increases interindividual variability of PK and pharmacodynamics, especially if body surface area (BSA)/weight influences VD and/or clearance.51 Thus, a PK simulation study demonstrated that a fixed dose of RTX would overdose small BSA patients whereas large BSA patients would be underdosed.51 In OBZ PK studies including CLL and NHL patients, the influence of BSA/weight on exposure remains controversial.52,53 Using higher doses at the beginning of treatment in order to quickly improve exposure and saturate targets was also suggested.50,54,55 Indeed, higher doses infused at days 1 and 8 (1600 mg) followed by infusions of 800 mg improved exposure and overall response rates (ORRs) in NHL, compared with lower doses (400 mg). In those patients, the influence of antigenic mass on OBZ exposure was evidenced at lower doses but not at higher doses.52 PK model-based simulation further demonstrated that a fixed dose of OBZ 1000 mg on days 1, 8, 15, and every 3 weeks thereafter (a total of 10 doses) provided satisfactory exposure for most patients with NHL and was selected for phase 3.52 In those phase 1/2 trials, FcγRIIIA-158VF polymorphism did not correlate with OBZ PK or objective response rate (ORR).50,54,55 In CLL patients, a phase 1/2 study suggested a dose-response relationship and an influence of tumor burden.56 This was further confirmed by retrospective analysis of PK data53 and also suggested by a prospective randomized phase 2 trial.57 Finally, the same dosing regimen as for NHL patients was applied for CLL. This dosing regimen, based on more rational development than that of RTX, will however always raise concerns when we compare OBZ and RTX results. Thus, until today, there is no demonstration that the clinical advantages of OBZ over RTX are not related to improved exposure.
What is the right OBZ comparator?
Undertaking a functional/pharmacological comparison between OBZ and RTX calls for extreme caution before drawing any conclusion. They indeed differ in 2 key characteristics: the nature of the IgG subclass (G1e5 vs standard IgG1), which modifies FcγR-dependent effector functions, and the angle of CD20 epitope recognition, which modifies the ability to trigger CDC and direct cytotoxicity. OBZ and RTX therefore differ in at least 3 possibly critical pharmacodynamics properties (Figure 1). Rigorous comparison, with the objective to identify which variation is responsible for which clinical effect, would require a step-by-step comparison, that is, testing a G1e5 variant of RTX in order to evaluate the contribution of Fc modification, testing a standard IgG1 version of OBZ to evaluate the clinical benefit of a standard type II anti-CD20 mAb, and eventually comparing OBZ to RTX, G1e5 RTX, and standard IgG1-OBZ. As far as we know, a G1e5 variant of RTX has never been clinically developed. Ocaratuzumab and ublituximab (Table 2), which are Fc-modified type I anti-CD20 mAbs (Figure 1B), could be considered as surrogate comparators; unfortunately, their clinical development is now interrupted or is not so far advanced,58 respectively. On the other side, because no naked Fc-unmodified type II anti-CD20 recombinant mAb (Figure 1C) is under clinical development, it would be necessary to compare OBZ to ocaratuzumab and ublituximab to definitely appreciate the actual advantage of type II anti-CD20 mAbs. Finally, to compare clinical results obtained with OBZ and RTX in order to understand how mAb modifications translate into clinical advantages deserved much attention and prudence.
How to interpret the results of OBZ clinical trials?
CLL: a role for FcγRIIIA-mediated mechanisms?
Unlike RTX, a phase 1/2 trial demonstrated a rapid clearance of B-cell CLL (B-CLL) circulating cells after the first OBZ infusion, with most patients experiencing a normal lymphocyte count before day 8.59 Similar results have been observed with the type I Fc-optimized mAb ublituximab.58 A large OBZ phase 3 trial included untreated CLL patients, randomized chlorambucil vs chlorambucil-RTX vs chlorambucil-OBZ.8,60 An increase in complete response rate, undetectable minimal residual disease, and PFS were observed with the OBZ arm compared with RTX. A significantly higher rate of infusion-related reactions (IRRs) was found with 20% of IRRs ≥ grade 3 with OBZ compared with 4% with RTX.
It was generally admitted that CLL gathered conditions for optimal CDC. The presence of tumor cells in the circulation together with complement proteins would create optimal conditions for anti-CD20 mAbs to initiate CDC. However, the reduced CD20 density in CLL as compared with NHL renders translocation in lipid rafts even more necessary,61,62 and that is what underlies the development of ofatumumab, currently approved in first-line and R/R CLL patients.63 A complement consumption was observed in CLL patients under RTX,64,65 contrary to what is observed with OBZ.56 The results of phase 3, demonstrating the superiority of OBZ over RTX in CLL, highly suggests that CDC is not necessary for anti-CD20 CLL therapy.
Until recently, no correlation was found between FcγRIIIA-158VF polymorphism and the outcome in R/R CLL patients treated with rituximab alone66 or associated with chemotherapy.67 It was then admitted that FcγRIIIA-dependent mechanisms did not play a critical role in RTX-mediated cytotoxicity of CLL cells. We demonstrated that FcγRIIIA-158VF polymorphism significantly correlated with RTX-induced B-cell peripheral depletion in untreated CLL patients receiving RTX alone,68 underlining the role of FcγRIIIA-mediated mechanisms in anti-CD20 CLL therapy. This would be particularly true in the context of low CD20 antigen density, which could require a better Fc-FcγRIIIA interaction,69 a situation encountered in FcγRIIIA-158VV patients68 or with Fc-optimized mAbs.
OBZ induces a higher rate of IRR ≥ grade 3 compared with RTX.60 Those IRRs occurred mainly at the time of the first infusion and were accompanied by an increase in cytokines (interleukin 6 [IL-6], IL-8, tumor necrosis factor-α) and interferon-α levels and by a concomitant decrease of circulating tumor cells and NK cells in peripheral blood with no increase of complement products.56 The lack of IRR recurrence or cytokine release during the subsequent infusions strongly suggests a relationship between cytokine release and IRR.59 The dramatic decrease of circulating B-CLL cells after the first infusion also suggests that cytokine release plays a role in the cytotoxic effect of OBZ. CD20 expression level by B-CLL cells, as well as FcγRIIIA expression by CD56+/FcγRIIIA+ cells and FcγRIIIA-158V/F polymorphism, were identified as the main risk factors of IRR.70 All of these results suggest that FcγRIIIA+ cell recruitment and activation take place in CLL patients after OBZ infusion and play a significant role in clinical activity and IRR occurrence. The increased incidence of IRR with OBZ, an Fc-optimized mAb having increased binding to FcγRIIIA, suggests therefore that FcγRIIIA-mediated mechanisms are the main mechanisms of OBZ action operating in CLL patients, at least after the first infusion. Interestingly, ublituximab also induces a high incidence of IRR in B-CLL patients.58 It remains to be determined whether the IRRs observed with these “FcγR engagers” are mechanistically similar to those commonly observed with the T-cell engagers (chimeric antigen receptor T cells, bispecific antibodies) and whether they would also benefit from IL-6Rα blockade therapy using tocilizumab,71 which is currently under investigation (NCT02336048). In this context, the role and the impact of the direct cell death induced by OBZ as a prototypical type II anti-CD20 mAb remains to be determined.
FL: always a suspected role for FcγRIIIA-mediated mechanisms?
A phase 2 OBZ trial demonstrated an ORR of 50% in FL patients,54 which was similar for the RTX refractory FL population. In a randomized phase 2 study, patients were randomly assigned to receive 4 weekly infusions of RTX (375 mg/m2) or OBZ (1000 mg) before maintenance.72 This is the only trial allowing a head-to-head comparison of these 2 mAbs in monotherapy. A significant increase of ORR was observed for OBZ but this potential advantage failed to translate in terms of PFS. In the GADOLIN trial including RTX refractory iNHL patients, OBZ-bendamustine was randomized with bendamustine alone, with the patients assigned in the OBZ-bendamustine arm receiving OBZ maintenance for 2 years.11 The authors demonstrated increased PFS in the iNHL population and increased PFS and overall survival in the FL population.10 OBZ-bendamustine, however, failed to increase ORR at the end of induction. The GALLIUM phase 3 study randomized standard RTX chemotherapy to OBZ chemotherapy (followed by maintenance for 2 years in each arm) in first-line FL.9 Three different chemotherapy regimens were allowed, but bendamustine represented 59% of treatments. Chemotherapy-associated OBZ significantly increased PFS compared with RTX, but again failed to improve ORR, even if a significant increase of undetectable minimal residual disease was observed at the end of induction with OBZ.73 The increased PFS suggests higher clinical activity of OBZ monotherapy compared with RTX, but the lack of significant advantage at the end of induction compared with RTX chemotherapy or chemotherapy alone,9,11 and the lack of clear advantage for OBZ in a head-to-head comparison, make drawing any definitive conclusion difficult.72
The importance of FcγRIIIA-mediated mechanisms in RTX clinical activity was first described in FL.2,3 By definition, FcγRIIIA-dependent mechanisms are highly dependent on the availability of effector cells at the tumor site, and this is even more important for OBZ, which could also better recruit myeloid cells expressing FcγRIIIB.23 The immune infiltrate has a prognostic impact on FL patients treated by chemotherapy74 and the initial adverse prognostic impact of tumor-associated macrophages found in the FL population treated by chemotherapy was abrogated74 and even inversed when the RTX-chemotherapy FL population was analyzed,75 arguing for a role of macrophages in anti-CD20 mAb activity.76,77 These effector cells could be missing during the period of chemotherapy, explaining in part the lack of increased ORR when OBZ is associated with chemotherapy.9,11 More generally, a limiting number of effector cells could contribute to limiting the clinical importance of these mechanisms, and hence the clinical impact of anti-CD20 mAb with an optimized Fc. To the contrary, it can be considered that there is a real clinical impact of Fc optimization, as in B-CLL, but that this impact is hampered by the loss of CDC activity of type II anti-CD20 mAb, resulting in a weaker advantage of OBZ over RTX than would be expected with Fc optimization only. Thus, it seems that anti-CD20 mAb therapy of FL should capitalize on both arms of the immune effectors that constitute the microenvironment, FcγR-expressing cells, and complement.
DLBCL: no advantage for Fc optimization?
An OBZ phase 1/2 study,55 including previously RTX-treated patients, demonstrated ORR of 30%, which was not influenced by OBZ exposure and was no different from that obtained with RTX.78 Untreated DLBCL patients were randomized to receive 8 cycles of either RTX-CHOP21 or OBZ-CHOP21 in the GOYA study.12 No difference was found in terms of ORR or PFS. Such results indicate that the improvement of FcγRIIIA-mediated mechanisms and/or enhancement of direct cytotoxicity do not translate into higher clinical activity in DLBCL. Likewise, excepting 1 study including the Chinese population,79 FcγRIIIA polymorphism does not influence the response to RTX in DLBCL, which is also characterized by a poor immune infiltrate, totally different from FL. In the GOYA study, a subanalysis suggested a slight increase of PFS with OBZ-CHOP compared with RTX-CHOP in germinal center DLBCL. Such a result was not found for activated B-cell DLBCL and unclassified DLBCL, and could suggest a more favorable immune context in germinal center DLBCL. The description of a different pattern of immune escape marker expression according to cell of origin could explain, in part, the ability of activated B-cell DLBCL to induce an exhaustion of antilymphoma cytotoxic T lymphocytes.80 Whether this environment could also negatively regulate innate immune effectors remains to be determined, but the global picture is that FcγRIIIA-mediated mechanisms are probably not so critical in DLBCL. As compared with FL, the natural history of DLBCL is also less influenced by complement regulatory proteins.81 We could therefore tentatively hypothesize that DLBCL are susceptible to RTX-induced CDC, and maybe more than FL. In this context, the gain of FcγRIIIA-mediated mechanisms and/or direct cytotoxicity that characterize OBZ would be annihilated by the loss of CDC. Maybe we will learn from ublituximab development whether Fc optimization without loss of CDC can provide better clinical results than RTX. The clinical development of PRO131921 is apparently stopped (Table 2), maybe unfortunately, as it would have provided a unique opportunity to evaluate in DLBCL an anti-CD20 mAb whose Fc was optimized for both FcγRIIIA and C1q binding.82
Conclusion
OBZ development has been justified by the importance of FcγRIIIA-mediated mechanisms in RTX clinical activity. A definitive conclusion on how increased affinity for FcγRIIIA translates into clinical activity is, however, obscured by a decrease of CDC and an increased direct cell death of OBZ compared with RTX. Moreover, the use of a different dosing regimen for RTX and OBZ further complicates any interpretation. Despite all of these issues, OBZ clearly improves the outcome of CLL and FL patients. The results obtained in CLL are of particular interest, as they provide new arguments for FcγRIIIA-mediated mechanisms of anti-CD20 mAbs in this disease. Results in FL confirm not only the interest for FcγRIIIA-mediated mechanisms in this histology, but also limitations of OBZ to induce higher response rates, some of them being possibly due to lack of CDC. The situation in DLBCL is deceptive, the possible gains of activity of OBZ appearing to be annihilated by the lack of CDC. By chance, RTX appears to be a mAb with equilibrated properties, leading to an “all-purpose” anti-CD20 mAb. Reinforcing one of these properties or another (Figure 1), mainly when it is done at the expense of another, exposes the new anti-CD20 mAb to be advantageous in some B-cell disorders only and to have restricted indications. The OBZ story taught us that reinforcing FcγRIIIA-dependent mechanisms can be very helpful when target cells expressed low levels of CD20, such as B-CLL, but that CDC should not be neglected. Whether FcγRIIIA-dependent mechanisms and CDC directly cooperate through mechanisms such as complement-dependent cell-mediated cytotoxicity, or whether they cooperate by recognizing, at the surface of target cells, anti-CD20 IgG1 monomers and hexamers, respectively, or they play a complementary role by acting on tumors at different times and places remains to be determined. Nevertheless, the OBZ story nicely demonstrates that designing mAbs with optimized and tailored properties is the future of naked mAbs, and that this has to be done step by step, with full clinical validation. Furthermore, anti-CD20 mAbs are now frequently used in combination with noncytotoxic drugs, especially those inhibiting the Bruton tyrosine kinase signaling pathway (ibrutinib, idelalisib) or BCL2 (venetoclax). These associations could theoretically offer safer treatments and avoid the detrimental effects of chemotherapy on anti-CD20 mAb therapy. However, some recent data indicate that ibrutinib interferes with the immune-mediated mechanisms induced by anti-CD20 mAbs.83,84 We now await studies of more selective Bruton tyrosine kinase inhibitors (acalabrutinib, tirabrutinib) on anti-CD20 mAb immune-mediated effects.
Acknowledgments
The authors thank Christophe Dumet for tracking information and generating the figures, and Alexandra Louault for refining the latter. The authors also thank Anne-Laure Gagez for reviewing the bibliography.
This work was supported by the French Higher Education and Research Ministry under the program Investissements d’Avenir grant agreement: LabEx MAbImprove ANR-10-LABX-53-01.
Authorship
Contribution: G.C. and H.W. wrote this paper.
Conflict-of-interest disclosure: G.C. received consultancy fees and honoraria from Roche and Celgene, and honoraria from Sanofi, Janssen, Gilead, and Bristol-Myers Squibb. H.W. declares no competing financial interests.
Correspondence: Guillaume Cartron, Département d’Hématologie Clinique, Centre Hospitalier Universitaire, 80 Avenue Augustin Fliche, 34095 Montpellier Cedex 05, France; e-mail: g-cartron@chu-montpellier.fr.
References
Author notes
G.C. and H.W. contributed equally to the content of this manuscript.