Key Points
Circulating sP-selectin shed from cell surfaces must dimerize to promote inflammation or coagulation.
Circulating sP-selectin is a consequence rather than a cause of cardiovascular disease.
Abstract
Leukocyte adhesion to P-selectin on activated platelets and endothelial cells induces shedding of the P-selectin ectodomain into the circulation. Plasma soluble P-selectin (sP-selectin) is elevated threefold to fourfold in patients with cardiovascular disease. Circulating sP-selectin is thought to trigger signaling in leukocytes that directly contributes to inflammation and thrombosis. However, sP-selectin likely circulates as a monomer, and in vitro studies suggest that sP-selectin must dimerize to induce signaling in leukocytes. To address this discrepancy, we expressed the entire ectodomain of mouse P-selectin as a monomer (sP-selectin) or as a disulfide-linked dimer fused to the Fc portion of mouse immunoglobulin G (sP-selectin-Fc). Dimeric sP-selectin-Fc, but not monomeric sP-selectin, triggered integrin-dependent adhesion of mouse leukocytes in vitro. Antibody-induced oligomerization of sP-selectin or sP-selectin-Fc was required to trigger formation of neutrophil extracellular traps. Injecting sP-selectin-Fc, but not sP-selectin, into mice augmented integrin-dependent adhesion of neutrophils in venules, generated tissue factor–bearing microparticles, shortened plasma-clotting times, and increased thrombus frequency in the inferior vena cava. Furthermore, transgenic mice that overexpressed monomeric sP-selectin did not exhibit increased inflammation or thrombosis. We conclude that elevated plasma sP-selectin is a consequence rather than a cause of cardiovascular disease.
Introduction
The selectins are adhesion receptors that initiate leukocyte movement from the vasculature into tissues at sites of inflammation, tissue injury, and immune surveillance.1,2 Each of the 3 selectins has an N-terminal C-type lectin domain, followed by an epidermal growth factor (EGF)-like domain, a series of consensus repeats, a transmembrane domain, and a short cytoplasmic tail. P-selectin is stored in membranes of α granules of platelets and Weibel-Palade bodies of endothelial cells. When mediators such as thrombin or histamine activate these cells, the granules fuse with the plasma membrane and P-selectin is rapidly redistributed to the cell surface. The lectin domain of P-selectin interacts reversibly with P-selectin glycoprotein ligand-1 (PSGL-1), a transmembrane homodimeric mucin on leukocytes. These interactions mediate leukocyte rolling on activated platelets and endothelial cells. P-selectin self-associates through its transmembrane domains into dimers and oligomers.3,4 Dimerization of P-selectin and PSGL-1 facilitates leukocyte rolling,5 which precedes activation of integrins that slow rolling and cause arrest. Numerous studies document how P-selectin initiates leukocyte recruitment during inflammation and supports hemostasis through platelet-leukocyte interactions.2,6 Dysregulated expression of P-selectin contributes to pathological inflammation and thrombosis in models of atherosclerosis, stroke, myocardial infarction, deep vein thrombosis, and other cardiovascular disorders.2,6
Soluble P-selectin (sP-selectin) circulates in plasma of both humans and mice. In humans, a minor portion of sP-selectin is derived from alternative messenger RNA splicing that removes the exon encoding the transmembrane domain.7-9 However, sP-selectin is primarily derived from proteolytic cleavage of the transmembrane protein, which releases a fragment comprising most of the ectodomain into the circulation. The ectodomain of P-selectin is quantitatively shed from activated platelets within 2 hours after they are infused into baboons or mice.10,11 Shedding is significantly reduced in PSGL-1–deficient mice,12 and basal plasma sP-selectin levels are much lower in PSGL-1–deficient mice.13 These results suggest that shedding requires leukocyte adhesion to activated platelets or endothelial cells through interactions of PSGL-1 with transmembrane P-selectin.
sP-selectin circulates at a concentration of 15 to 100 ng/mL in wild-type (WT) mice and in healthy human subjects.3,14 sP-selectin levels are elevated in patients with atherosclerosis, hypertension, hyperlipidemia, myocardial infarction, and postangioplasty restenosis.15 Even in acute settings such as myocardial infarction, sP-selectin increases no more than fourfold over control levels. However, the consistent elevation of sP-selectin in patients with cardiovascular disease has attracted interest in its use as a biomarker. For example, modestly elevated sP-selectin levels in apparently healthy women are associated with increased risk of future myocardial infarction, stroke, and cardiovascular death.16
Significantly, the current consensus is that circulating sP-selectin directly contributes to cardiovascular disease. Three lines of evidence support this view. First, knock-in mice that express transmembrane P-selectin lacking the cytoplasmic domain (ΔCT mice)14 have approximately threefold to fourfold elevated levels of circulating sP-selectin and exhibit enhanced susceptibility to thrombosis, stroke, and atherosclerosis.17,18 Second, injecting P-selectin-Fc chimeras into mice causes release of procoagulant leukocyte microparticles, shortens plasma-clotting times, and increases β2 integrin–dependent adhesion of neutrophils to platelets and endothelial cells in vitro and in vivo.17,19-21 Third, plasma from patients with peripheral arterial occlusive disease activates neutrophil integrins in vitro, but immunodepletion of P-selectin reverses this effect.22
Nevertheless, other evidence suggests that circulating sP-selectin is not prothrombotic or proinflammatory. In contrast to dimeric or multimeric P-selectin on cell surfaces, the ectodomain of human sP-selectin remains monomeric at high concentrations.3 As measured by surface plasmon resonance, the Kd for binding of monomeric human sP-selectin to PSGL-1 is ∼1 μM.23 This corresponds to ∼100 μg/mL for a relative molecular mass of 100 000 for sP-selectin.11 In ΔCT mice, the moderately elevated level of sP-selectin in plasma is still 100-fold lower than the Kd for binding to PSGL-1, assuming similar affinities in mice and humans.14,18 In humans, plasma sP-selectin levels are far below the Kd; even in disease states, none exceeds 500 ng/mL and most are fivefold to 30-fold lower.15,16 Thus, sP-selectin, if monomeric in plasma, would bind to only a small percentage of PSGL-1 molecules on circulating leukocytes, limiting signaling. In vitro, concentrations of dimeric sP-selectin that saturate PSGL-1 on leukocytes induce signaling, whereas saturating concentrations of monomeric sP-selectin do not.20,24,25 In most reports, the amount of dimeric P-selectin-Fc injected into mice likely achieves plasma concentrations of 10 to 30 μg/mL,17,19,20 well above those of circulating sP-selectin in ΔCT mice or in humans.
Establishing a direct contribution of sP-selectin to cardiovascular disease could have major clinical impact. If circulating sP-selectin is harmful, drugs that block proteolytic release from cell membranes could reduce cardiovascular risk. On the other hand, shedding of the ectodomain to reduce the P-selectin density on membranes may limit inflammation and thrombosis. If so, drugs that block cleavage of P-selectin could be harmful. It is therefore important to determine whether sP-selectin is a cause or consequence of cardiovascular disease. To address this unresolved issue, we directly compared the effects of injecting monomeric and dimeric sP-selectin in vivo. We also characterized transgenic mice that overexpress monomeric sP-selectin to chronically elevate levels in plasma. Our results strongly suggest that monomeric sP-selectin shed from cell surfaces into the circulation does not promote inflammation and coagulation.
Methods
The supplemental Methods (available on the Blood Web site) provide details for reagents; mass spectrometry; sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE), immunoprecipitation, and western blotting; flow cytometry; static cell adhesion assay; intravital microscopy; clotting assay; quantitation of microparticles bearing tissue factor; measurement of neutrophil extracellular traps (NETs); the deep vein thrombosis model; and plasma cytokine/chemokine assay.
Mice
All protocols were approved by the institutional animal care and use committee of the Oklahoma Medical Research Foundation.
C57BL/6J (WT) mice and P-selectin–deficient (Selp−/−) mice in the C57BL/6J background26 were from The Jackson Laboratory. We generated transgenic mice overexpressing sP-selectin (Tg-sP-selectin). A pBluescript KS(−) vector containing the promoter-enhancer for the mouse Alb gene27 (a gift from Richard Palmiter, University of Washington) was digested with NotI and KpnI to release the insert, which was blunted using Klenow fragment. A pcDNA 3.1(−) vector containing a complementary DNA (cDNA) insert encoding mouse sP-selectin was linearized with XhoI, blunted with Klenow fragment, and dephosphorylated with alkaline phosphatase. The albumin promoter-enhancer was ligated 5′ to the sP-selectin cDNA in this vector. Positive clones were identified with EcoRV digestion and DNA sequencing. A 5096-bp transgene fragment containing the promoter-enhancer fused to the sP-selectin cDNA and a 3′ bovine growth hormone poly(A) sequence was excised with NheI and NaeI. The purified transgene was microinjected into a C57BL/6 mouse single-cell embryo at the Transgenic Mouse/Gene Targeting Core Facility at Emory University School of Medicine. Two founder lines with an integrated transgene were confirmed by genotyping. One founder line that consistently overexpressed sP-selectin over multiple generations was used. Although Tg-sP-selectin mice were generated in the C57BL/6 background, littermates not expressing transgenic sP-selectin were still used as controls.
Mice aged 8 to 12 weeks were used in all protocols except for deep vein thrombosis experiments, where mice were aged 14 to 16 weeks. Male mice were used for intravital microscopy, clotting assays, and deep vein thrombosis experiments. Mice of both sexes were used for all other experiments.
Recombinant protein synthesis and purification
To express mouse sP-selectin, a cDNA encoding the entire extracellular domain of mouse P-selectin fused to a C-terminal 8-residue epitope for the Ca2+-dependent monoclonal antibody (mAb) HPC428 was inserted into the XhoI and EcoRV restriction sites of pcDNA 3.1(−). To express sP-selectin-Fc, the Fc domain of mouse immunoglobulin G2a (IgG2a) was amplified from the pFUSE-mIgG2a-Fc1 vector (InvivoGen) and ligated into the EcoRI and BclI sites of the pEE14.1 expression vector (Lonza Biologics). The insert encoding sP-selectin fused to the HPC4 epitope was subcloned into this vector. The sP-selectin and sP-selectin-Fc vectors were separately transfected into CHO-K1 cells. Stable cell lines expressing sP-selectin or sP-selectin-Fc were selected using G418 or methionine sulphoximine, respectively, and were further selected for high protein expression by enzyme-linked immunosorbent assay (ELISA). sP-selectin or sP-selectin-Fc was purified from conditioned medium by immunoaffinity chromatography using mAb HPC4 and dialyzed into phosphate-buffered saline (PBS), pH 7.4, containing 0.5 mM CaCl2.
Gel filtration
Mouse sP-selectin or sP-selectin-Fc (100 µL at 2 mg/mL) was applied to a HiLoad 16/60 Superdex 200 column equilibrated with PBS containing 0.5 mM CaCl2 on an AKTA-FPLC system (GE Healthcare Life Sciences). Fractions (0.5 mL) were collected at a flow rate of 0.5 mL per minute. Peak fractions were detected at A280 nm. The void volume (39.8 mL) was determined using blue dextran (2000 kDa). The column was calibrated with a high-molecular-weight gel filtration kit supplemented with carbonic anhydrase and cytochrome C. Human sP-selectin (100 µL at 1 mg/mL), which was previously shown to be an extended monomer,3 was used as additional calibration for mouse sP-selectin. Mouse IgG (100 µL at 0.5 mg/mL) was used as calibration for mouse sP-selectin-Fc.
ELISA for sP-selectin
Mouse blood was collected by heart or facial-vein puncture into EDTA-containing tubes and centrifuged at 1500g for 15 minutes. The resulting plasma was centrifuged at 15 000g for 5 minutes and either used immediately or frozen at −80°C. Immulon-2HB 96-well microtiter plates coated overnight at 4°C with 100 µL of anti-mouse P-selectin mAb 5H1 (2 μg/mL in sodium bicarbonate buffer, pH 9.2) were washed and blocked with 2% bovine serum albumin for 1 hour. All incubations were at room temperature. Plasma samples diluted in PBS containing 0.1% Triton X-100 and 0.1% bovine serum albumin were added to wells and incubated for 2 hours. Wells were washed and incubated with 100 µL of 50 ng/mL biotinylated goat anti-P-selectin antibody for 2 hours. Alternatively, mAb HPC4 biotinylated with the EZ-Link Sulfo-NHS-Biotin kit was incubated at 1 μg/mL for 2 hours. The wells were washed, and streptavidin–horseradish peroxidase (1:5000) was added and incubated for 30 minutes. After a final wash, stabilized chromogen-H2O2 solution was added. An equal volume of 1 N H2SO4 was added to stop color development. The plates were read at 570 nm in a FLUOstar Omega microplate reader (BMG Labtech). sP-selectin or sP-selectin-Fc levels in plasma were quantified using recombinant sP-selectin or sP-selectin-Fc, respectively, as standard. The linear range of the assay was 10 pg/mL to 10 ng/mL, with a detection limit of 1 pg/mL.
Circulating half-life measurements
Recombinant mouse sP-selectin or sP-selectin-Fc (10-20 µg) was injected intravenously or intraperitoneally into Selp−/− mice. Blood was collected at different times after injection, and plasma levels of injected protein were quantified using ELISA. The percentage of circulating sP-selectin remaining was calculated relative to its concentration 2.5 hours after injection. The circulating half-life was determined by nonlinear regression using a 2-phase decay statistical analysis model.29
Surface plasmon resonance
Surface plasmon resonance was performed on a Biacore 3000 instrument. The running buffer was PBS, pH 7.4, 1.0 mM CaCl2, 0.005% Tween 20. Biotinylated 2-GSP-6, a glycosulfopeptide that comprises the N-terminal binding site for P-selectin on human PSGL-1,30 was captured on a research grade, streptavidin-coated sensor chip pretreated according to the manufacturer’s instructions. A control sensor was mock-treated but without capturing biotinylated 2-GSP-6. Mouse sP-selectin or sP-selectin-Fc was diluted in running buffer. Alternatively, they were added to plasma from Selp−/− mice diluted 1/4 in running buffer. Increasing concentrations of sP-selectin or sP-selectin-Fc were injected over 2-GSP-6 and control surfaces. Samples in buffer were injected at 40 μL per minute for 30 seconds; samples in diluted plasma were injected at 10 μL per minute for 2 minutes. These injection times were sufficient to reach equilibrium binding. Specific binding was measured by subtracting nonspecific binding to the control surface. Equilibrium binding data were analyzed by nonlinear curve fitting of the Langmuir binding isotherms using BiaEvaluation software.
Statistics
Data are presented as mean ± standard error of the mean (SEM) unless written otherwise. Statistical differences were determined by a 2-tailed Student t test or 1-way analysis of variance with the Bonferroni test for multiple comparisons. Frequency of thrombus formation between different groups was determined using the contingency table and the χ2 test. Prism 7 (GraphPad software) was used for all statistical analysis. P < .05 was considered statistically significant.
Results
Mouse dimeric sP-selectin binds with higher avidity to PSGL-1 than mouse monomeric sP-selectin
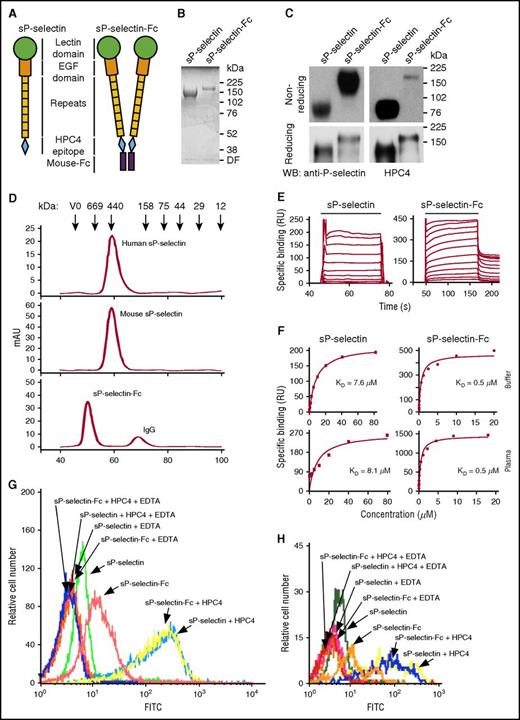
We expressed recombinant monomers (sP-selectin) and dimers (sP-selectin-Fc) of the mouse P-selectin ectodomain in transfected CHO cells. The monomeric sP-selectin contained the lectin domain, the EGF domain, and all 8 consensus repeats, followed by an 8-residue epitope for the Ca2+-dependent mAb HPC4 (Figure 1A). The C terminus of this construct was fused to the Fc portion of mouse IgG2a to produce sP-selectin-Fc, a disulfide-linked dimer (Figure 1A). Monomers and dimers isolated by immunoaffinity chromatography were homogeneous by Coomassie blue staining following SDS-PAGE (Figure 1B). Mass spectrometry assigned a molecular weight of 93.4 kDa to monomeric sP-selectin, from which an extinction coefficient was derived to determine protein levels in solution. Both monomers and dimers were recognized by a mAb to a lectin-domain epitope and by mAb HPC4 (Figure 1C), confirming preservation of the entire ectodomain. Recombinant human sP-selectin was previously shown to be an extended monomer.3 Human and mouse sP-selectin coeluted as extended monomers during gel filtration, whereas mouse sP-selectin-Fc eluted as a dimer (Figure 1D).
Mouse dimeric sP-selectin-Fc binds with higher avidity to PSGL-1 than mouse monomeric sP-selectin. (A) Schematics of mouse sP-selectin and sP-selectin-Fc. (B) SDS-PAGE of sP-selectin and sP-selectin-Fc under reducing conditions, followed by Coomassie blue staining. (C) Western blots (WB) of sP-selectin and sP-selectin-Fc electrophoresed under reducing and nonreducing conditions, probed with polyclonal anti-P-selectin IgG or mAb HPC4. (D) Elution profiles of the indicated proteins applied to a Superdex 200 column, with fractions detected by absorbance at 280 nm. The column was calibrated by the elution profile of the indicated proteins. (E) Overlays of increasing concentrations of sP-selectin or sP-selectin-Fc binding to 2-GSP-6, a surrogate for PSGL-1, on a sensor surface. The horizontal line indicates when sP-selectin or sP-selectin-Fc in running buffer was injected. (F) Nonlinear curve fits of specific binding data for proteins injected in running buffer (Buffer) or in plasma diluted 1/4 in running buffer (Plasma). The data represent the mean ± standard deviation (SD) from 3 experiments. (G-H) Flow cytometry of mouse neutrophils (G) and monocytes (H) incubated with FITC-labeled sP-selectin or sP-selectin-Fc with or without oligomerization by mAb HPC4, in Ca2+-containing buffer or in buffer containing EDTA. The data in panels B-D and G-H are representative of 3 experiments. DF, dye front; mAU, milliabsorbance unit; RU, resonance unit.
Mouse dimeric sP-selectin-Fc binds with higher avidity to PSGL-1 than mouse monomeric sP-selectin. (A) Schematics of mouse sP-selectin and sP-selectin-Fc. (B) SDS-PAGE of sP-selectin and sP-selectin-Fc under reducing conditions, followed by Coomassie blue staining. (C) Western blots (WB) of sP-selectin and sP-selectin-Fc electrophoresed under reducing and nonreducing conditions, probed with polyclonal anti-P-selectin IgG or mAb HPC4. (D) Elution profiles of the indicated proteins applied to a Superdex 200 column, with fractions detected by absorbance at 280 nm. The column was calibrated by the elution profile of the indicated proteins. (E) Overlays of increasing concentrations of sP-selectin or sP-selectin-Fc binding to 2-GSP-6, a surrogate for PSGL-1, on a sensor surface. The horizontal line indicates when sP-selectin or sP-selectin-Fc in running buffer was injected. (F) Nonlinear curve fits of specific binding data for proteins injected in running buffer (Buffer) or in plasma diluted 1/4 in running buffer (Plasma). The data represent the mean ± standard deviation (SD) from 3 experiments. (G-H) Flow cytometry of mouse neutrophils (G) and monocytes (H) incubated with FITC-labeled sP-selectin or sP-selectin-Fc with or without oligomerization by mAb HPC4, in Ca2+-containing buffer or in buffer containing EDTA. The data in panels B-D and G-H are representative of 3 experiments. DF, dye front; mAU, milliabsorbance unit; RU, resonance unit.
We used surface plasmon resonance to measure binding of each protein to a glycosulfopeptide that comprises the N-terminal binding site on human PSGL-1.30 sP-selectin bound with rapid association and dissociation kinetics (Figure 1E). sP-selectin-Fc also bound rapidly but dissociated much slower, consistent with its dimeric structure. Nonlinear curve fitting of the equilibrium-binding isotherms indicated that sP-selectin-Fc bound with ∼20-fold higher affinity than sP-selectin due to its higher avidity (Figure 1F). Incubation of either protein in plasma from P-selectin–deficient (Selp−/−) mice did not alter binding, indicating that plasma does not oligomerize the P-selectin ectodomain (Figure 1F). We then used flow cytometry to compare binding of mouse sP-selectin and sP-selectin-Fc to PSGL-1 on mouse leukocytes. Equal concentrations of fluorescein isothiocyanate (FITC)-conjugated sP-selectin or sP-selectin-Fc were incubated with mouse neutrophils and monocytes (Figure 1G-H). Both sP-selectin and sP-selectin-Fc exhibited Ca2+-dependent binding, with higher levels for sP-selectin-Fc. Adding mAb HPC4 to oligomerize sP-selectin and sP-selectin-Fc induced significantly greater binding, likely due to more FITC-conjugated P-selectins in an oligomer binding to each PSGL-1 molecule and to a higher percentage of P-selectin–occupied PSGL-1 molecules on the cell surface.
sP-selectin must dimerize or oligomerize to induce leukocyte signaling in vitro
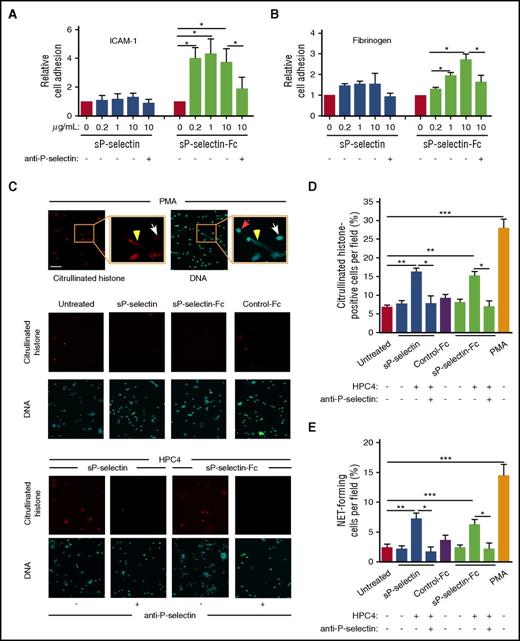
Some agonists induce β2 integrin–dependent adhesion of leukocytes to ICAM-1 or fibrinogen. As observed previously,20 increasing concentrations of sP-selectin-Fc, but not sP-selectin, increased adhesion of mouse bone marrow leukocytes (mostly neutrophils) to immobilized ICAM-1 (Figure 2A) or fibrinogen (Figure 2B).
sP-selectin must dimerize or oligomerize to induce leukocyte signaling in vitro. (A-B) Static adhesion of mouse leukocytes to immobilized ICAM-1 or fibrinogen, in the presence of the indicated concentration of sP-selectin or sP-selectin-Fc with or without blocking anti-P-selectin mAb. (C) Fluorescence micrographs of purified mouse neutrophils incubated with PMA (positive control, top row); with buffer (Untreated), sP-selectin, sP-selectin-Fc, or control IgG (Control-Fc) (middle 2 rows); or with sP-selectin or sP-selectin-Fc oligomerized with mAb HPC4 in the presence or absence of neutralizing anti-P-selectin mAb (bottom 2 rows). After incubation, the cells were fixed and stained with anticitrullinated histone IgG or with Sytox Green to label DNA. The insets in the top row are higher magnifications. The red arrow marks a neutrophil without staining for citrullinated histones and with staining for DNA only in the nucleus. The white arrow marks a neutrophil stained for citrullinated histones and DNA only in the nucleus. The yellow arrowhead marks a neutrophil with extracellular staining for both citrullinated histones and DNA, indicating NET release. Original magnification ×20; inset magnification 2.7-fold. Scale bar, 50 μm. (D-E) Quantification of cells stained for citrullinated histones or forming NETs (stained for both citrullinated histones and extracellular DNA). The data in panels A, B, D, and E represent the mean ± SEM from 5 experiments. *P < .05, **P < .01, ***P < .001.
sP-selectin must dimerize or oligomerize to induce leukocyte signaling in vitro. (A-B) Static adhesion of mouse leukocytes to immobilized ICAM-1 or fibrinogen, in the presence of the indicated concentration of sP-selectin or sP-selectin-Fc with or without blocking anti-P-selectin mAb. (C) Fluorescence micrographs of purified mouse neutrophils incubated with PMA (positive control, top row); with buffer (Untreated), sP-selectin, sP-selectin-Fc, or control IgG (Control-Fc) (middle 2 rows); or with sP-selectin or sP-selectin-Fc oligomerized with mAb HPC4 in the presence or absence of neutralizing anti-P-selectin mAb (bottom 2 rows). After incubation, the cells were fixed and stained with anticitrullinated histone IgG or with Sytox Green to label DNA. The insets in the top row are higher magnifications. The red arrow marks a neutrophil without staining for citrullinated histones and with staining for DNA only in the nucleus. The white arrow marks a neutrophil stained for citrullinated histones and DNA only in the nucleus. The yellow arrowhead marks a neutrophil with extracellular staining for both citrullinated histones and DNA, indicating NET release. Original magnification ×20; inset magnification 2.7-fold. Scale bar, 50 μm. (D-E) Quantification of cells stained for citrullinated histones or forming NETs (stained for both citrullinated histones and extracellular DNA). The data in panels A, B, D, and E represent the mean ± SEM from 5 experiments. *P < .05, **P < .01, ***P < .001.
sP-selectin-Fc was reported to stimulate mouse neutrophils to form extracellular traps (NETs).31 Citrullination of DNA-associated histones is a prerequisite for NET formation.32 Phorbol myristate acetate (PMA), a well-documented NET agonist,31,32 induced mouse neutrophils to citrullinate histones, as measured by immunofluorescence microscopy, and to release NETs, as measured by staining of both citrullinated histones and DNA outside cells (Figure 2C-E). We found that neither sP-selectin nor sP-selectin-Fc caused mouse neutrophils to citrullinate histones or to release NETs (Figure 2C-E). However, oligomerization of either protein with mAb HPC4 significantly increased histone citrullination and NET release, which was prevented by neutralizing anti-P-selectin mAb. We cannot explain why we could not replicate the reported induction of NETs by sP-selectin-Fc without additional crosslinking.31 Regardless, our data demonstrate that P-selectin must dimerize or oligomerize to induce signaling in leukocytes.
sP-selectin and sP-selectin-Fc have long half-lives in the circulation
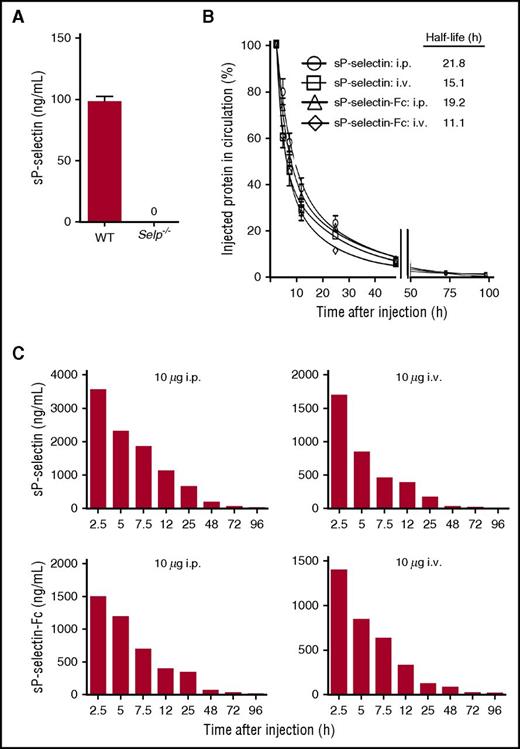
Using recombinant mouse sP-selectin or sP-selectin-Fc as standard, we developed 2 ELISAs that measured mouse P-selectin over a broad concentration range. In both ELISAs, P-selectin was captured on an immobilized anti-P-selectin mAb that recognizes the lectin domain. One ELISA used biotinylated goat anti-P-selectin antibody to measure native or recombinant P-selectin. The other ELISA used biotinylated mAb HPC4 to measure only recombinant sP-selectin or sP-selectin-Fc. Using the first ELISA, we found that plasma levels of native sP-selectin in WT mice were ∼100 ng/mL, consistent with previous reports (Figure 3A). The ELISA did not detect P-selectin in plasma of Selp−/− mice, confirming its specificity. Injecting sP-selectin or sP-selectin-Fc significantly increased plasma P-selectin; the injected protein could be distinguished from native P-selectin with the second ELISA. Both sP-selectin and sP-selectin-Fc had long plasma half-lives after IV or intraperitoneal injection (Figure 3B). This made it possible to sustain high plasma levels of monomeric or dimeric sP-selectin in vivo (Figure 3C).
sP-selectin and sP-selectin-Fc have long half-lives in the circulation. (A) Plasma levels of sP-selectin in WT or Selp−/− mice. (B) Relative plasma levels of sP-selectin or sP-selectin-Fc in Selp−/− mice at the indicated times after injecting 10 to 20 μg of the indicated protein intravenously (i.v.) or intraperitoneally (i.p.). The circulating half-life was determined using a 2-phase linear regression fit. (C) Representative absolute plasma concentrations of sP-selectin or sP-selectin-Fc in Selp−/− mice at the indicated times after injecting 10 μg of the indicated protein i.p. or i.v. The data in panels A and B represent the mean ± SEM from 3 to 5 experiments.
sP-selectin and sP-selectin-Fc have long half-lives in the circulation. (A) Plasma levels of sP-selectin in WT or Selp−/− mice. (B) Relative plasma levels of sP-selectin or sP-selectin-Fc in Selp−/− mice at the indicated times after injecting 10 to 20 μg of the indicated protein intravenously (i.v.) or intraperitoneally (i.p.). The circulating half-life was determined using a 2-phase linear regression fit. (C) Representative absolute plasma concentrations of sP-selectin or sP-selectin-Fc in Selp−/− mice at the indicated times after injecting 10 μg of the indicated protein i.p. or i.v. The data in panels A and B represent the mean ± SEM from 3 to 5 experiments.
Injecting sP-selectin-Fc, but not sP-selectin, promotes inflammation and coagulation in vivo
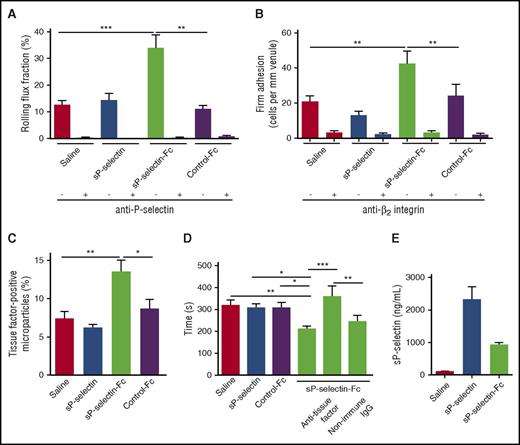
Surgical trauma during mobilization of the cremaster muscle induces P-selectin–dependent rolling of neutrophils in postcapillary venules.33 Because little chemokine is expressed in this model, few rolling neutrophils undergo β2 integrin–dependent arrest. We found that injecting sP-selectin-Fc, but not sP-selectin, increased the numbers of neutrophils rolling on P-selectin (Figure 4A) and the numbers of neutrophils that arrested through β2 integrins (Figure 4B).
Injecting sP-selectin-Fc, but not sP-selectin, promotes inflammation and coagulation in vivo. (A-B) Number of rolling cells and arrested cells in trauma-stimulated venules of WT mice injected IV with saline, sP-selectin, sP-selectin-Fc, or control-Fc in the presence or absence of blocking anti-P-selectin mAb or anti-β2 integrin mAb. (C) Percentage of tissue factor–positive microparticles in plasma of WT mice 22 hours after intraperitoneal injection of saline, sP-selectin, sP-selectin-Fc, or control-Fc. (D) Plasma-clotting times of WT mice 22 hours after intraperitoneal injection of saline, sP-selectin, sP-selectin-Fc, or control-Fc, in the presence or absence of blocking anti-tissue factor IgG or nonimmune IgG. (E) Plasma levels of sP-selectin or sP-selectin-Fc 22 hours after intraperitoneal injection. The data in panels A and B represent the mean ± SEM from 15 to 20 venules from 4 to 5 mice in each group. The data in panels C-E represent the mean ± SEM from 7 to 12 experiments in each group. *P < .05, **P < .01, ***P < .001.
Injecting sP-selectin-Fc, but not sP-selectin, promotes inflammation and coagulation in vivo. (A-B) Number of rolling cells and arrested cells in trauma-stimulated venules of WT mice injected IV with saline, sP-selectin, sP-selectin-Fc, or control-Fc in the presence or absence of blocking anti-P-selectin mAb or anti-β2 integrin mAb. (C) Percentage of tissue factor–positive microparticles in plasma of WT mice 22 hours after intraperitoneal injection of saline, sP-selectin, sP-selectin-Fc, or control-Fc. (D) Plasma-clotting times of WT mice 22 hours after intraperitoneal injection of saline, sP-selectin, sP-selectin-Fc, or control-Fc, in the presence or absence of blocking anti-tissue factor IgG or nonimmune IgG. (E) Plasma levels of sP-selectin or sP-selectin-Fc 22 hours after intraperitoneal injection. The data in panels A and B represent the mean ± SEM from 15 to 20 venules from 4 to 5 mice in each group. The data in panels C-E represent the mean ± SEM from 7 to 12 experiments in each group. *P < .05, **P < .01, ***P < .001.
Previous studies showed that injecting sP-selectin-Fc causes leukocytes to release tissue factor–bearing microparticles into the circulation and shortens tissue factor–dependent clotting times in plasma.19 We confirmed these effects of sP-selectin-Fc (Figure 4C-D). However, injecting sP-selectin failed to generate tissue factor–expressing microparticles or shorten clotting times (Figure 4C-D), even though plasma sP-selectin was higher than sP-selectin-Fc (Figure 4E).
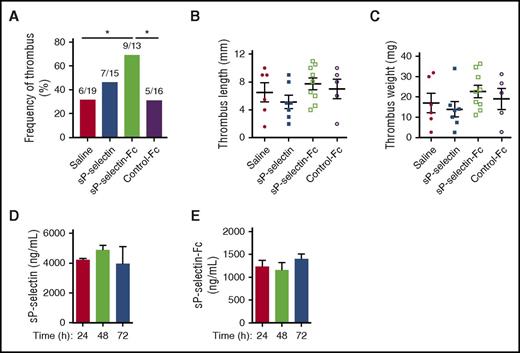
Tissue factor is a major contributor to thrombosis in the inferior vena cava of mice.34 We measured the incidence and size of thrombi in the inferior vena cava 48 hours after ligation to reduce blood flow. Injection of sP-selectin-Fc significantly increased the incidence of thrombi (Figure 5A), with a trend toward increased thrombus length and weight that did not reach statistical significance (Figure 5B-C). In contrast, injection of sP-selectin affected neither thrombus incidence nor size (Figure 5A-C), even though plasma sP-selectin was much higher than sP-selectin-Fc before and throughout the 48 hours after flow reduction (Figure 5D-E).
Injecting sP-selectin-Fc, but not sP-selectin, increases the frequency of deep vein thrombi. WT mice were injected intraperitoneally every 24 hours for 3 days (time 0, 24 hours, or 48 hours) with saline, sP-selectin, sP-selectin Fc, or control-Fc. Twenty-two hours after the first injection, the inferior vena cava was ligated to induce thrombosis. After 72 hours (48 hours after ligation), the incidence, size, and weight of thrombi were measured. (A) Frequency of thrombus. For each experimental group, the number of mice forming thrombi relative to the total number of mice is shown. (B) Thrombus length. (C) Thrombus weight. (D-E) Plasma levels of sP-selectin or sP-selectin-Fc at the indicated time after injection. The data represent the mean ± SEM from 10 to 16 experiments in each group. *P < .05.
Injecting sP-selectin-Fc, but not sP-selectin, increases the frequency of deep vein thrombi. WT mice were injected intraperitoneally every 24 hours for 3 days (time 0, 24 hours, or 48 hours) with saline, sP-selectin, sP-selectin Fc, or control-Fc. Twenty-two hours after the first injection, the inferior vena cava was ligated to induce thrombosis. After 72 hours (48 hours after ligation), the incidence, size, and weight of thrombi were measured. (A) Frequency of thrombus. For each experimental group, the number of mice forming thrombi relative to the total number of mice is shown. (B) Thrombus length. (C) Thrombus weight. (D-E) Plasma levels of sP-selectin or sP-selectin-Fc at the indicated time after injection. The data represent the mean ± SEM from 10 to 16 experiments in each group. *P < .05.
Chronic elevation of circulating sP-selectin does not promote inflammation and coagulation
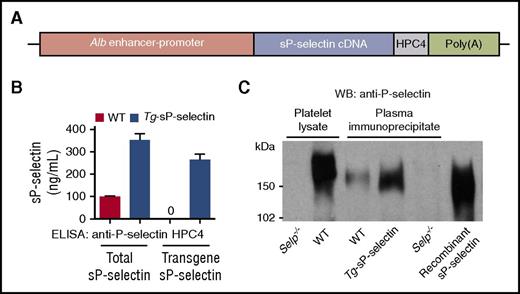
To determine whether a chronic increase in plasma sP-selectin exerts adverse effects, we made transgenic mice that express the mouse P-selectin ectodomain with the C-terminal epitope for mAb HPC4, under control of the Alb enhancer/promoter (Figure 6A). One founder was bred into the C57BL/6J background for >9 generations. The mice were healthy, fertile, and had normal lifespans. The mice stably expressed ∼3.5-fold higher levels of sP-selectin in plasma, most of which contained the HPC4 epitope encoded by the transgene (Figure 6B). We probed P-selectin immunoprecipitated from plasma by western blotting (Figure 6C). More sP-selectin was immunoprecipitated from plasma of transgenic mice than of WT mice. No P-selectin was immunoprecipitated from plasma of Selp−/− mice, confirming its specificity. Plasma sP-selectin from WT and transgenic mice comigrated with recombinant sP-selectin and migrated faster than transmembrane P-selectin from platelet lysates, consistent with its smaller size (Figure 6C).
Generation of transgenic mice (Tg-sP-selectin) expressing chronically elevated plasma levels of sP-selectin. (A) Schematic of the sP-selectin transgene. (B) Plasma levels of total sP-selectin (measured with anti-P-selectin IgG) or transgenic sP-selectin (measured with mAb HPC4) in WT or Tg-sP-selectin mice. The data represent the mean ± SEM from 8 experiments in each group. (C) Western blots (WB) of P-selectin from platelet lysates or from immunoprecipitates of plasma from mice of the indicated genotype, or of recombinant sP-selectin (right lane). The data are representative of 5 experiments.
Generation of transgenic mice (Tg-sP-selectin) expressing chronically elevated plasma levels of sP-selectin. (A) Schematic of the sP-selectin transgene. (B) Plasma levels of total sP-selectin (measured with anti-P-selectin IgG) or transgenic sP-selectin (measured with mAb HPC4) in WT or Tg-sP-selectin mice. The data represent the mean ± SEM from 8 experiments in each group. (C) Western blots (WB) of P-selectin from platelet lysates or from immunoprecipitates of plasma from mice of the indicated genotype, or of recombinant sP-selectin (right lane). The data are representative of 5 experiments.
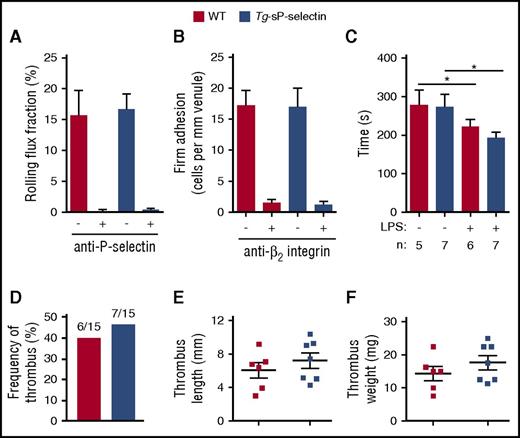
Elevated plasma sP-selectin did not induce detectable systemic inflammation in unchallenged transgenic mice. A multiplex assay revealed normal plasma levels of 23 cytokines and chemokines, including interleukin β (IL-1β), tumor necrosis factor, granulocyte-macrophage colony-stimulating factor, and CXCL1 (Table 1). Furthermore, inflammatory cytokines and chemokines were equivalently elevated in WT and transgenic mice 4 hours after injection with lipopolysaccharide (Table 1). Similar numbers of neutrophils rolled on P-selectin and arrested through β2 integrins in trauma-stimulated venules of WT and transgenic mice (Figure 7A-B). Tissue factor–dependent plasma-clotting times were equivalent in both genotypes (Figure 7C). Injecting lipopolysaccharide into mice of both genotypes shortened clotting times to the same degree. The incidence and size of thrombi in the flow-restricted inferior vena cava was similar in WT and transgenic mice (Figure 7D-F). Thus, chronic elevation of circulating sP-selectin did not promote inflammation or coagulation in these models.
Chronic elevation of circulating sP-selectin does not promote inflammation and coagulation. (A-B) Number of rolling cells and arrested cells in trauma-stimulated venules of WT or Tg-sP-selectin mice in the presence or absence of blocking anti-P-selectin mAb or anti-β2 integrin mAb. (C) Plasma-clotting times of WT or Tg-sP-selectin mice without challenge or 4 hours after intraperitoneal injection of lipopolysaccharide (LPS). (D-F) Frequency of thrombus, thrombus length, and thrombus weight in WT or Tg-sP-selectin mice 48 hours after ligation of inferior vena cava. The data in panels A and B represent the mean ± SEM from 11 to 15 venules from 5 to 6 mice in each group. The data in panels D-F represent the mean ± SEM from 15 experiments in each group. *P < .05.
Chronic elevation of circulating sP-selectin does not promote inflammation and coagulation. (A-B) Number of rolling cells and arrested cells in trauma-stimulated venules of WT or Tg-sP-selectin mice in the presence or absence of blocking anti-P-selectin mAb or anti-β2 integrin mAb. (C) Plasma-clotting times of WT or Tg-sP-selectin mice without challenge or 4 hours after intraperitoneal injection of lipopolysaccharide (LPS). (D-F) Frequency of thrombus, thrombus length, and thrombus weight in WT or Tg-sP-selectin mice 48 hours after ligation of inferior vena cava. The data in panels A and B represent the mean ± SEM from 11 to 15 venules from 5 to 6 mice in each group. The data in panels D-F represent the mean ± SEM from 15 experiments in each group. *P < .05.
Discussion
We have shown that circulating sP-selectin must dimerize or oligomerize to promote inflammation or coagulation in vivo. Injecting dimeric sP-selectin-Fc into mice to raise plasma levels consistently induced leukocyte adhesion and leukocyte-dependent procoagulant activity. However, injecting monomeric sP-selectin failed to exert these effects, even at plasma concentrations >30-fold higher than basal levels of circulating sP-selectin. Furthermore, chronic elevation of monomeric sP-selectin in plasma of transgenic mice did not promote inflammation or coagulation.
Our in vivo results extend previous in vitro studies that sP-selectin must dimerize or oligomerize to trigger signaling in leukocytes.20,24,25 We confirmed that sP-selectin-Fc, but not sP-selectin, promoted integrin-dependent adhesion of leukocytes in vitro. Furthermore, antibody-induced oligomerization of sP-selectin or sP-selectin-Fc was required to induce neutrophils to form NETs. Transmembrane P-selectin transduces signals through multivalent engagement of PSGL-1 molecules when leukocytes adhere to activated platelets or endothelial cells.2 Both PSGL-1 and P-selectin dimerize in cell membranes,3,4,35 which enhances signaling. In contrast, little or no signaling is likely when monomeric sP-selectin binds to PSGL-1. We found that monomeric mouse sP-selectin, like human sP-selectin,23 not only bound rapidly to PSGL-1 but also dissociated rapidly. Plasma did not alter binding affinity or kinetics, strongly suggesting that circulating sP-selectin remains monomeric. Given its affinity, the modestly elevated (threefold to fourfold) levels of sP-selectin found in cardiovascular disorders would permit binding to only a few PSGL-1 molecules on circulating leukocytes.
Two studies have suggested that monomeric sP-selectin triggers signals in leukocytes. In the first study, monomeric sP-selectin was reported to cause exposure of procoagulant phosphatidylserine on the surface of monocytes.36 The authors provided limited documentation of the sP-selectin and did not confirm its monomeric state. Furthermore, optimal signaling was observed at an sP-selectin concentration >25-fold higher than basal plasma levels, much higher than those seen in inflammatory or thrombotic disorders. In the second study, monomeric sP-selectin at concentrations observed in disease was reported to induce integrin-dependent adhesion of leukocytes.22 However, the same group later used the same concentrations of dimeric sP-selectin-Fc to activate integrins on leukocytes.21 It is difficult to reconcile how the same concentration range for signaling could be observed for both monomers and dimers. This group noted fourfold higher sP-selectin levels in plasma from patients with peripheral arterial occlusive disease.22 Immunodepletion of P-selectin from patient plasma was shown to reverse its ability to stimulate leukocyte adhesion in vitro. However, the authors did not determine whether immunodepletion removed P-selectin–expressing platelet microparticles, which are elevated in cardiovascular disorders and can interact with leukocytes.37,38 Plasma or circulating microparticles from patients with cardiovascular disease could also contain other mediators that stimulate leukocytes.
The most cited evidence that circulating sP-selectin promotes inflammation and thrombosis comes from studies of ΔCT mice. These mice express P-selectin that lacks the cytoplasmic domain but retains the transmembrane domain.14 They have increased circulating procoagulant microparticles derived from monocytes,17 and their neutrophils are primed to release NETs.31 The ΔCT mice are more susceptible to thrombosis, strokes, and atherosclerosis.18 These effects have been ascribed to the threefold to fourfold increase in circulating sP-selectin. In contrast, we found no excessive inflammation or thrombosis in our transgenic mice, which have a similar threefold to fourfold increase in circulating sP-selectin. We suggest an alternative interpretation for the phenotype of ΔCT mice. The deletion of the cytoplasmic domain prevents sorting of P-selectin from the trans-Golgi network into the Weibel-Palade bodies of endothelial cells. As a result, newly synthesized P-selectin is transported directly to the cell surface.14 Deletion of the cytoplasmic domain also prevents endocytosis of P-selectin into clathrin-coated pits.39 These combined effects cause constitutive expression of P-selectin on endothelial cells, which increases basal rolling of leukocytes. Rolling not only increases PSGL-1–dependent shedding of sP-selectin into plasma, but also favors signaling through multivalent binding of P-selectin to PSGL-1. Increased basal rolling may further permit chemokines on endothelial cells to amplify leukocyte responses. This could explain why thrombosis and atherosclerosis are more prominent in ΔCT mice than in WT mice injected with sP-selectin-Fc.18,40,41 Thus, the shedding of sP-selectin in ΔCT mice may be a byproduct rather than a cause of vascular disease.
Although circulating sP-selectin is a widely used biomarker for inflammatory and thrombotic disorders, our results challenge the prevailing view that it directly contributes to disease. The P-selectin ectodomain likely circulates as a monomer after it is shed from cell surfaces and must dimerize to promote inflammation or coagulation. Shedding of the ectodomain, by reducing the P-selectin density on activated platelets and endothelial cells, may instead function to limit leukocyte adhesion and signaling.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Richard Palmiter for reagents.
This work was supported by National Institutes of Health grants HL034363 (National Heart, Lung, and Blood Institute) and GM114731 (National Institute of General Medical Sciences).
Authorship
Contribution: S.R.P., P.M.-D., N.Z., A.G.K., and B.S. conducted experiments; and S.R.P., P.M.-D., and R.P.M. designed experiments, analyzed data, and wrote the paper, with input from all authors.
Conflict-of-interest disclosure: R.P.M. is cofounder of Selexys Pharmaceuticals, now part of Novartis AG, and of Tetherex Pharmaceuticals. The remaining authors declare no competing financial interests.
Correspondence: Rodger P. McEver, Cardiovascular Biology Research Program, Oklahoma Medical Research Foundation, 825 NE 13th St, Oklahoma City, OK 73104; e-mail: rodger-mcever@omrf.org.