To the editor:
Inherited bone marrow failure syndromes include entities such as Fanconi anemia, Diamond-Blackfan anemia, Shwachman-Diamond syndrome, severe congenital neutropenia, and telomere biology disorders/dyskeratosis congenita (TBDs/DKC). These are potentially devastating conditions that affect multiple organ systems, and patients with these conditions benefit from specialized, multidisciplinary care. Pregnancy outcomes in patients with inherited bone marrow failure syndromes are poorly defined.1,,,,,,-8 As more patients with inherited bone marrow failure syndromes survive to adulthood, additional information regarding potential barriers to pregnancy, anticipated pregnancy outcomes, and optimal pregnancy management is needed. In this letter, we report our experience with pregnancy outcomes in inherited bone marrow failure syndromes.
To identify adults with inherited bone marrow failure syndromes who might have experienced pregnancy, we performed a search of the Partners HealthCare Research Patient Data Registry, a centralized clinical data registry that includes information on a large number of patients seen within the Partners HealthCare system (Institutional Review Board protocol 2017P000675/PHS).9 To capture patient pregnancies, we limited the search to females seen by an obstetrician or gynecologist. Data between January 1991 and December 2016 were examined. Genetic information was not available for most patients because they were seen at Partners HealthCare sites primarily for obstetrical/gynecological reasons; however, nearly all of the patients had been diagnosed with inherited bone marrow failure syndromes by experienced outside hematologists at institutions such as Boston Children’s Hospital. It is worth noting that, for a number of these syndromes, the genetic cause of a large fraction of the cases remains unknown even after extensive genetic testing, and the diagnosis remains a clinical one.10
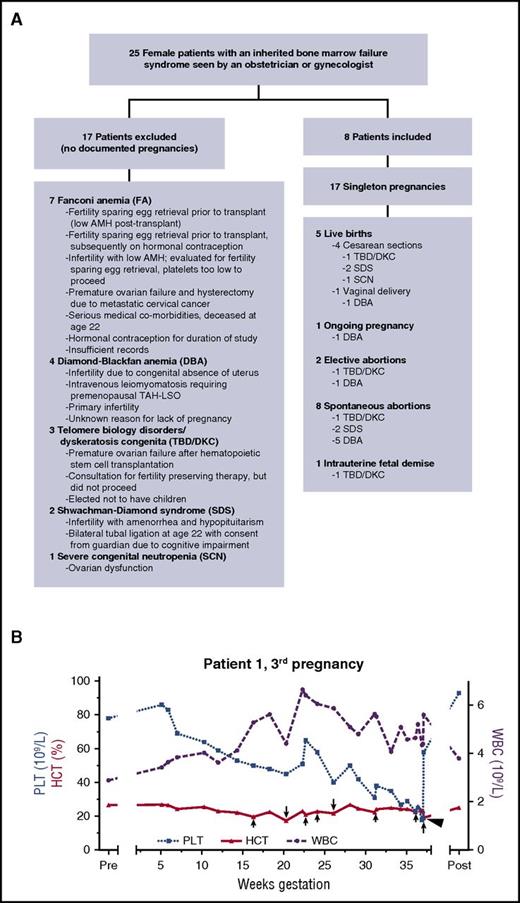
Of 25 patients initially identified by our search, 17 were excluded (Figure 1A); there were 17 pregnancies in the remaining 8 women (Figure 1A; Table 1). These included 5 successful live births (4 cesarean sections and 1 spontaneous vaginal delivery), 8 spontaneous abortions (4 with documented fetal cardiac activity prior to demise), 2 elective abortions, 1 intrauterine fetal death, and 1 ongoing pregnancy. Only singleton gestations were documented, all but one of which was conceived without in vitro fertilization. No patients were known to be related. No patients became pregnant after stem cell transplantation. No patients who experienced pregnancy have been previously reported in the literature, except that it is unknown if Patient 8 reported her pregnancy to the Severe Chronic Neutropenia International Registry.1
Study schematic and blood count trends in 1 successful pregnancy. (A) Schematic of evaluable patients. (B) Blood count parameters during the third pregnancy of Patient 1, who had short telomeres and a heterozygous TERC mutation (TBD/DKC). Red blood cell transfusions are indicated by arrows, with longer arrows representing 2 units transfused and shorter arrows representing 1 unit transfused. A single platelet transfusion is represented by an arrowhead. HCT, hematocrit; PLT, platelet count; Post, 4 months postpartum; Pre, 2 weeks before predicted conception; WBC, white blood cell count.
Study schematic and blood count trends in 1 successful pregnancy. (A) Schematic of evaluable patients. (B) Blood count parameters during the third pregnancy of Patient 1, who had short telomeres and a heterozygous TERC mutation (TBD/DKC). Red blood cell transfusions are indicated by arrows, with longer arrows representing 2 units transfused and shorter arrows representing 1 unit transfused. A single platelet transfusion is represented by an arrowhead. HCT, hematocrit; PLT, platelet count; Post, 4 months postpartum; Pre, 2 weeks before predicted conception; WBC, white blood cell count.
Infertility was strikingly common. No pregnancies occurred in the 7 patients with Fanconi anemia (Figure 1A). Premature ovarian failure at a young age may be partly to blame. Decreased levels of anti-Müllerian hormone (AMH) were observed both pre– and post–stem cell transplantation, which has been reported in a small number of patients.11 It is not yet clear if AMH deficiency is a primary or secondary defect in patients with Fanconi anemia,12 but AMH knockout mice suffer from early depletion of primordial follicles.13 Given the high rate of premature ovarian failure in women with Fanconi anemia, it would be reasonable to consider oocyte preservation at a young age in these patients.
The optimal management of cytopenias during pregnancy in patients with inherited bone marrow failure syndromes has not been defined, but transfusion support was successful in a few of our cases. For Patient 1, who had short telomeres and a heterozygous TERC mutation previously reported to be disease-causing,14 anemia and thrombocytopenia worsened early in each of her 3 pregnancies and then rebounded (Figure 1B and data not shown). Epoetin alfa did not prevent the need for red blood cell transfusion in Patient 1’s first pregnancy, which ended in intrauterine fetal death at 20 weeks of gestation. Patient 1’s third pregnancy was supported by transfusion of red blood cells and platelets. She developed transient severe hypertension in the setting of a red blood cell transfusion at 36 weeks of gestation and was admitted for monitoring until delivery 1 week later. Patient 3, who had Shwachman-Diamond syndrome, was supported with platelet transfusions during her fourth pregnancy. Although 2 doses of romiplostim at 5 mg/kg were administered during this pregnancy, one late in gestation and one after delivery, there was no clear effect on the platelet count. There are very few data regarding the use of romiplostim in pregnancy, but it was not administered earlier in gestation due to safety concerns.15 For Patient 8, who had severe congenital neutropenia, the administration of granulocyte-colony stimulating factor (G-CSF) during pregnancy appeared to be safe, consistent with 1 prior report.1 However, despite maintaining an absolute neutrophil count >1.0 × 109/L, she still suffered infectious complications: chorioamnionitis, mastitis, and poor wound healing at her cesarean section site.
For one of the patients, there was a particularly informative experience with false-negative genetic testing. Patient 1, who had very short telomeres, carried a heterozygous TERC mutation. Although the commercial laboratory that performed her initial testing detected the mutation, their software program was unable to annotate it and therefore filtered it out so that it was not reported. This is more likely to occur with genes encoding short RNAs, such as TERC, because the determination of variant pathogenicity for genes that are not translated is more challenging. Even when pathogenic variants are not detected, the reporting of variants of uncertain significance, which was not done for Patient 1, can be very helpful to clinicians. The possibility of false-negative sequencing results when clinical suspicion for a mutation is high should lead to a discussion with the sequencing facility that conducted the initial testing. Also, given rapid improvements in the annotation of the human genome, recuration of variants in previously tested patients is essential.
Two patients with TBDs/DKC, one with a TERC mutation (Patient 1) and one without an identifiable mutation (Patient 2), were noted to have blocked fallopian tubes. Patient 1 conceived spontaneously after fallopian tube recanalization despite a low AMH level, but she was unable to breastfeed due to poor milk production. Whether fallopian tube blockage and impaired lactation are more common in patients with short telomeres requires the study of a greater number of patients. A fibrotic process leading to fallopian tube blockage could be envisioned, because patients with TBDs/DKC sometimes develop pulmonary fibrosis and/or liver cirrhosis.16,17 Low AMH levels in patients with TBDs/DKC have been described in 1 report.12
Our experience supports the need for multidisciplinary collaboration in caring for patients with inherited bone marrow failure syndromes who are pregnant or desire pregnancy. In addition to the team of hematologists, maternal-fetal medicine specialists, fertility specialists, and neonatologists, input from other specialists may be helpful. For instance, patients with Shwachman-Diamond syndrome, in whom gastrointestinal malabsorption is common, may benefit from consultation with a nutritionist or gastroenterologist given the possibility of poor weight gain during pregnancy. Taken together, the data presented in this letter suggest that infertility and pregnancy loss are significant issues for patients with inherited bone marrow failure syndromes; however, healthy live births can occur despite pregnancy complications related to the severity of the underlying disease.
Authorship
Acknowledgments: J.M.G. was supported by training grant T32HL116324 and career development program award K12HL087164 from the National Institutes of Health, National Heart, Lung, and Blood Institute.
Contribution: J.M.G. designed the study, performed chart reviews, generated the first draft of the manuscript and table, guided the generation of the figure, and incorporated feedback from the other authors; M.M.A., K.J.G., A.P., J.M.C., R.I.H., N.B., A.S., and E.S.G. contributed to the design of the study and revised the manuscript; R.Y.-F. and E.L. generated part of the figure and revised the manuscript; N.T.C. and M.N.D. contributed to the design of the study and reviewed the manuscript; and N.A.S performed chart reviews, helped with the generation of the table and figure, and revised the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: John M. Gansner, Hematology Division, Department of Medicine, Brigham and Women’s Hospital, Harvard Medical School, 75 Francis St, Boston, MA 02115; e-mail: jgansner@bwh.harvard.edu.