Abstract
The incidence of pediatric venous thromboembolism (VTE) has been increasing significantly over the past decade in part as a result of increased recognition of this serious disorder but more so because of the increased use of central venous catheters and other technological advancements involved in the care of ill children. Management of pediatric VTE is a complex undertaking, considering that the vast majority of children who develop this complication have serious underlying medical disorders. Although the incidence is rising, in comparison with adults, this remains a relatively rare disorder, and as such, large-scale clinical trials have not been completed, rendering management decisions to be based on extrapolation from adult data and the experience of the treating physician. Clearly, both are fraught with problems. Thus, day-to-day management remains more art than science until such time that the results from clinical trials (many of which are under way) become available. This edition of “How I Treat” describes the author’s experience in managing 3 common scenarios that one may encounter in pediatric thrombosis and suggests a logical approach to such situations. Furthermore, the author provides 3 algorithms to help guide management decisions.
Introduction
Pediatric venous thromboembolism (VTE) is an ever-increasing phenomenon leading to significant complications and death in children and touching every aspect of pediatric medicine.1 There are several notable differences between VTE in children and adults. First, the majority of thrombotic events in children are caused by central venous catheters (CVC), and as a corollary to this fact, there is an overrepresentation of upper-extremity thrombosis.2 Second, nearly all episodes of VTE in children are provoked. This author has cared for ∼2500 patients with VTE and can recall only 1 case which was truly idiopathic. Third, children of all ages and sizes can develop VTE resulting in a great variety of the types of patients seen from premature neonates weighing less than 1 kg to obese adolescents weighing over 150 kg and from highly complex patients with a dozen or more comorbid conditions to otherwise healthy children who acquire the antiphospholipid antibody syndrome. The diversity of patients leads to numerous challenges from diagnosis to treatment. Examples include the difficulty in obtaining a Doppler ultrasound examination in a neonate who is so small that the probes are nearly as big as the patient to the risk and inconvenience of anesthesia that is required for many younger children to obtain a useful magnetic resonance imaging (MRI) scan for evaluation of cerebral venous sinus thrombosis (CSVT). The variety in not just size but developmental status of children leads to challenges in dosing and administration of anticoagulant medications. For example, warfarin is available only as a tablet and cannot be compounded into a liquid formulation, making it even more difficult to use than it already is in the youngest patients. In addition, administering subcutaneous anticoagulants is very challenging in children, given the associated pain and the need for their parents to serve in unpleasant roles. In addition to the above, another crucial difference between adult and pediatric VTE is the lack of evidence-based medicine approaches due to the paucity of high-quality data from clinical trials. Although there are numerous higher- quality clinical trials under way, particularly as it relates to the use of direct anticoagulants (DOACs), it will be several years more before these trials mature and begin to yield results. Thus, this edition of “How I Treat” aims to provide clear and practical recommendations for managing several typical scenarios that occur in pediatric VTE that can be applied today in spite of the lack of randomized clinical trials to support decision making. Figures 1 to 3 provide algorithms for anticoagulation options divided by age and decision-tree algorithms for catheter-related VTE in general (presented as a neonate in case 1) and for idiopathic lower-extremity DVT (presented in case 3). Each case will first present the challenges and choices facing the treating physician, followed by the opinions of the author as to how he would treat each patient. The scenarios are presented in age order.
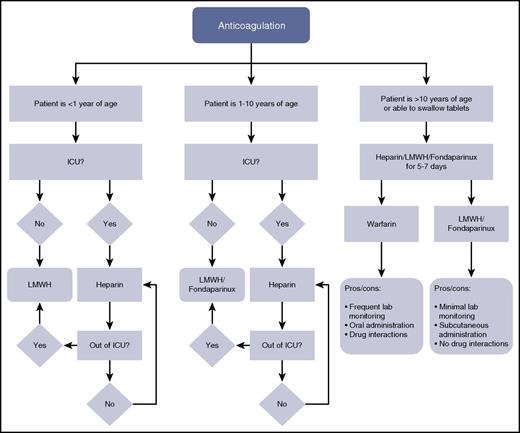
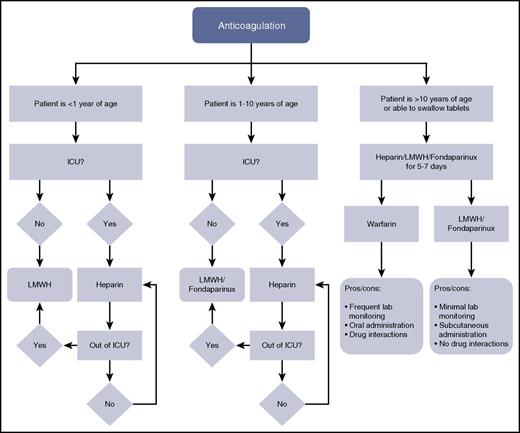
Algorithm for anticoagulation selection by age. Of note, because direct oral anticoagulants are not yet in wide clinical use, they are not included in this algorithm. ICU, intensive care unit; LMWH, low-molecular-weight heparin.
Algorithm for anticoagulation selection by age. Of note, because direct oral anticoagulants are not yet in wide clinical use, they are not included in this algorithm. ICU, intensive care unit; LMWH, low-molecular-weight heparin.
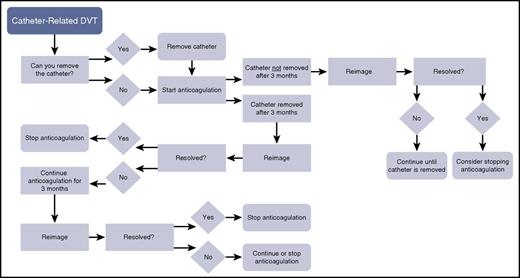
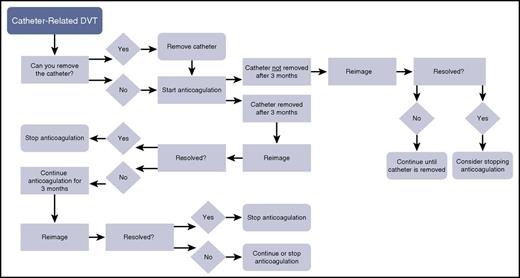
A decision-tree algorithm for catheter-related venous thrombosis. DVT, deep vein thrombosis.
A decision-tree algorithm for catheter-related venous thrombosis. DVT, deep vein thrombosis.
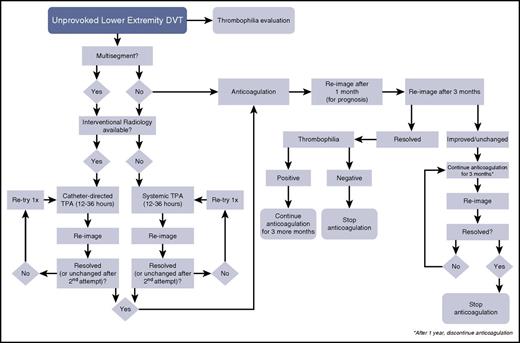
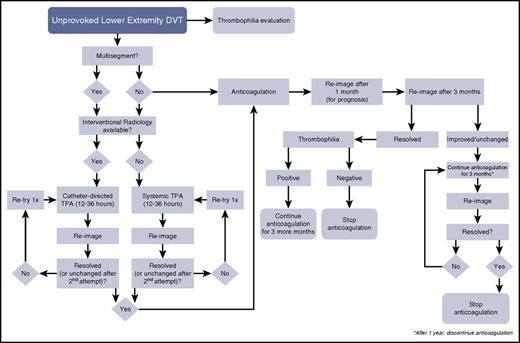
A decision-tree algorithm for idiopathic lower-extremity DVT. TPA, tissue plasminogen activator.
A decision-tree algorithm for idiopathic lower-extremity DVT. TPA, tissue plasminogen activator.
Case 1: neonatal VTE
Challenges and choices
A 3-week-old born at 27 weeks gestation develops a right iliofemoral DVT associated with a peripherally inserted central catheter, which was placed in order to manage common postnatal complications. A pediatric hematologist is consulted to provide recommendations for managing the VTE. The major questions for the consultant are as follows: (1) Should the catheter be removed? (2) Is a thrombophilia evaluation warranted? (3) Should this patient receive thrombolytic therapy? (4) Does this patient need anticoagulation and if so, which one, at what dose, and for how long?
First, what recommendations should be made with respect to the catheter? Ordinarily, this would be an easy answer; however, the consultant needs to ask one vital question: “If I recommend removing this catheter and you do so, will you need to place another one?” An affirmative answer should question the wisdom of removing one CVC only to place a second one unless, of course, the catheter is no longer functional, in which case the decision to remove it is clear. The advantage of removing the catheter is clearly the removal of the source of the thrombus and may help with resolution of the clot; however, the disadvantage is forcing the patient to undergo another invasive procedure and putting the patient (who already has demonstrated a propensity to catheter-related thrombosis) at risk for a second thrombotic event.
The second question was whether a thrombophilia evaluation should be performed. One might prefer to have more information before answering, such as whether there is a strong family history for thrombosis, and although it would be prudent to inquire about this, it is not clear whether this should affect the decision to pursue a detailed genetic work-up. One might also ask whether the results of the evaluation would affect the management of the patient before making that decision.
The third and fourth questions revolved around treatment. First and foremost is whether there are contraindications to thrombolysis or anticoagulation. Certainly, neonates, and especially premature ones, have an increased risk for bleeding complications. For instance, does this neonate have intraventricular hemorrhage, and if so, what grade is it and has it resolved? No one would disagree with not offering thrombolysis or anticoagulation to this patient if the risk of exacerbating an active intracranial bleed exists. Assuming such a risk does not exist, the next question is whether treatment is warranted at all. The answer to this question must take into account the answer to the first question—whether the CVC is going to be removed or not. Could removing the CVC be sufficient for treating this VTE? As will be repeated throughout this review, an evidence-based answer is not available. Finally, if treatment beyond catheter removal is suggested, should thrombolysis be considered? To answer this question, one should ask what the advantages of rapid clot resolution are in this patient, and if an advantage exists, does it offset the added risk for bleeding? With regard to anticoagulation, what options exist for this type of patient? It would be important to know the patient’s prognosis and course of care to best address this question because some anticoagulants (unfractionated heparin) are only administered to hospitalized patients and in some hospitals only for those in intensive care units.
How I would treat this patient
It seems obvious that this patient would continue to require a CVC for many days if not weeks, and as such, I would not advocate removing the catheter only for another one to be placed elsewhere. Regarding a thrombophilia evaluation, there are several reasons not to perform one and significant challenges if one chooses to do so. In my view, there is a clear and obvious cause for this event, and whatever treatment is pursued is highly unlikely to be affected by any positive findings. Second, interpretation of results for tests of natural inhibitors to anticoagulation (antithrombin, proteins C and S) would be very difficult, given the physiologically low levels of these proteins, which are even lower in premature neonates. Although other tests, such as those for factor V Leiden or the prothrombin mutation, would not be subject to interpretation, their value in this setting is limited and could, in fact, lead to more stress and confusion for the parents. Thus, I would not recommend a thrombophilia evaluation in this patient. The only caveat would be to test for antithrombin levels if achieving therapeutic anticoagulation with antithrombin-dependent anticoagulants is problematic. While it would be very challenging, if not impossible, to diagnose an inherited antithrombin deficiency, the results could provide insight as to why a patient might be “resistant” to anticoagulation and could lead to a change in choice of anticoagulant to a direct thrombin inhibitor such as bivalirudin or argatroban.3 Finally, with respect to therapy, because I would not advocate removal of the catheter, I would certainly treat the patient with anticoagulation, assuming there was no contraindication. Thrombolysis in premature neonates carries with it a high risk for intracranial hemorrhage4 and would not offer much more benefit in this scenario than would anticoagulation. I would recommend using either unfractioned heparin (UFH) or LMWH in this patient, depending on his or her expected length of stay, the availability of subcutaneous tissue (not a given in a premature neonate), and the ability to procure blood for therapeutic drug monitoring easily. Because this patient has a CVC and an expected length of stay of probably several weeks, I would treat with UFH until shortly prior to discharge, at which time I would switch to LMWH to provide enough time to find the proper dose and to give the parents time to learn subcutaneous injection. It should be noted that management with UFH may create some challenges, such as interruption of anticoagulation, if other medications that are incompatible with heparin are being infused through the central venous access device intermittently and complicating therapeutic drug monitoring, because samples drawn from the central venous access device in which the heparin is infusing could lead to false results. Thus, the hematologist should discuss these possible challenges with the primary team and determine whether or not UFH is still the best option. If these challenges cannot be overcome, treatment with LMWH, which is subcutaneous, can solve both problems. With respect to the duration of treatment, there are no clinical trials that would support any choice; however, proceeding from the most recent (2012) American College of Chest Physicians (ACCP) recommendations,5 one could treat for 6 weeks to 3 months. I would favor 6 weeks of treatment if the catheter is removed quickly (within a few days) but would recommend 3 months of therapy if the catheter remained in place for several more weeks. The Kids Duration of Therapy for Thrombosis study is under way, which is evaluating a shorter (6 week) course for low-risk patients.6
Case 2: cerebral venous sinus thrombosis
Challenges and choices
Within the context of VTE in children, CSVT is relatively uncommon; however, it can occur in a number of clinical scenarios, including children with cancer, particularly with acute lymphoblastic leukemia, and children with infections of the head and neck. Although other conditions can result in CSVT, for the sake of this case, I focus on the most common situation, which is that of a head and neck infection.
The case involves an 8-year-old with chronic sinusitis who has been on and off antibiotics for nearly a year and presents with worsening headache and double vision for 3 weeks. An MRI demonstrates extensive thrombosis of left cavernous sinus with extension into the left sigmoid, left transverse, and superior sagittal sinus. The major questions for this case are as follows. (1) Is there a role for mechanical interventions such as surgery or stenting? (2) Is a thrombophilia evaluation warranted? (3) Should this patient receive thrombolytic therapy? (4) Does this patient need anticoagulation and, if so, which one, at what dose, and for how long? (5) Are there ancillary therapies that should be considered?
With respect to surgery, there are several issues. First, does this child need surgery to manage the condition that led to the thrombosis? Second, are there interventional procedures that should be considered as either primary measures or supportive measures to manage this patient? There is no question that a multidisciplinary approach is required for such a patient, including an infectious disease specialist, an otorhinolaryngologist, a neurologist, and a neurosurgeon. Managing the sinusitis is an important part of treatment, perhaps not so much for the current thrombosis, which has clearly extended well beyond the nasal sinuses, but rather to ensure that there will not be a recurrence, not to mention preventing other complications that could arise from this infection. Although primary management of the infection is best left to other experts, the hematologist in addition to advising about anticoagulation must make it clear that treatment of the infection is a crucial aspect of treating the clot. With respect to management of the CSVT itself, there are no data supporting the use of surgery nor stenting in children, and the role of the hematologist is to convey the message that medical management is usually highly successful and that invasive (and risky) procedures are not warranted solely for managing the CSVT.
With respect to a thrombophilia evaluation, this scenario is less clear than is the first one. Although there is a clear provoking condition, chronic sinusitis is quite common; CSVT is much rarer, invoking the question, “why this child?” Obtaining a detailed family history may facilitate making this decision. As for thrombolytic therapy, the data on its use in CSVT in children are scant, making its use risky and without a clear benefit-to-risk ratio. Having said that, the cases described in the published case reports are for patients who either presented with severe complications (coma) or were not responsive to anticoagulation, so more aggressive approaches are not to be completely dismissed.7,8
Anticoagulation is the mainstay of management of CSVT and is based on extrapolation from clinical trials in adults with a Cochrane review of those trials supporting the benefit of anticoagulation.9 Pediatric trials have not been performed. Thus, though some discussion regarding the need for anticoagulation in this scenario may be productive, especially with the other consultants, it would be unusual not to treat this patient with anticoagulation unless there was a strong contraindication. An important point to mention is the fact that finding evidence of intracranial hemorrhage in a patient with CSVT is not unusual and is likely secondary to the venous hypertension in the cerebral veins draining into the thrombosed sinuses, signaling more advanced disease. These hemorrhages are not a contraindication to anticoagulation; to the contrary, they make anticoagulation that much more important to initiate and to do so expeditiously. In adults, there is limited evidence from clinical trials demonstrating the benefits of anticoagulation, which have been nicely reviewed.10 In pediatrics, the vast experience with anticoagulation is with UFH, LMWH, and warfarin. Because UFH is given by continuous infusion, it would be used (if at all) only during hospitalization. Alternatively, LMWH could be the initial anticoagulant prescribed, and it or warfarin can be used for outpatient management. Regarding which anticoagulant to recommend is less of an issue with the thrombus itself and more to do with logistical concerns such as ease of oral administration, ease of subcutaneous administration, ease of obtaining blood samples to perform therapeutic drug monitoring, and patient and parent choice. It should be noted that the published data regarding DOACs in children are extremely limited; thus they cannot be recommended at this time for a patient of this age.11,12 A key issue with regard to management of CSVT is the duration of therapy. Again, no clinical trial evidence exists to support decision making, and options include short courses of 3 months to longer courses of 6 to 12 months. A logical endpoint to therapy is complete resolution of both the thrombus as assessed by MRI and symptoms. This often takes well beyond 3 months in the author’s experience. Whether or not it is prudent to stop anticoagulation prior to reaching these 2 important milestones is not known.
The last issue has to do with ancillary therapies. It is not uncommon for children with such extensive CSVT to have symptoms of intracranial hypertension, which could continue for many months. This may be due to damage to the arachnoid villi, which are present in all the dural sinuses, though they are most prominent in the superior sagittal sinus. This symptom can be debilitating, leading to visual disturbances, severe pain precluding the ability to go to school, an inability to focus, and memory loss leading to poor learning. Thus management of intracranial hypertension may be required as an important adjunct. Consultation with a pediatric neurologist knowledgeable in CSVT is helpful if available; however, pediatric hematologists caring for such patients should also be comfortable with managing at least the straightforward cases. Reduction of intracranial hypertension can be managed medically with acetazolamide or procedurally with repeated lumbar punctures; however, given the relative ease of oral medications combined with the issues of performing lumbar puncture in patients on anticoagulation, clearly the easiest first option is prescribing acetazolamide. More serious neurologic complications such as seizures should be managed in conjunction with a pediatric neurologist.
How I would treat this patient
As soon as the diagnosis is verified and precluding any contraindications to anticoagulation, I would initiate treatment immediately upon diagnosis with UFH. This option allows for rapid anticoagulation and the early achievement of a therapeutic level. Neither interventional procedures nor thrombolysis are indicated except in the most severe cases, such as those with herniation, coma, and status epilepticus. It would not be unreasonable to consider LMWH as well, but the advantages of UFH in a patient who will likely be hospitalized for at least several days make it my preferred choice. Once the patient is more stable and plans for discharge are being made, conversion to LMWH, fondaparinux, or warfarin can begin. As has been stated, the decision regarding which anticoagulant rests more with logistical issues such as the ability of the child to swallow tablets and be comfortable with repeated venipuncture to assess the degree of anticoagulation. For a child who can swallow tablets and would have no difficulties with repeated venipuncture, I would opt for warfarin. Otherwise, either LMWH or fondaparinux can be used with the major advantage of fondaparinux being its once-daily dosing.13,14 The next question, is how long to treat? Over the past nearly 20 years and with approximately 200 patients treated, I have developed a high degree of respect for the seriousness of this condition and as such have adopted a rather conservative approach after noting numerous problems by stopping “early.” At this time, I favor the approach of continuing anticoagulation until there is complete resolution of symptoms and complete radiologic resolution. This often takes a minimum of 6 months and typically 12 months. For most patients, this endpoint is reached with patience and perseverance. For those who develop permanent neurologic sequelae, this approach may need to be modified. Obviously, this is a less objective endpoint, and consultation with a pediatric neurologist can be helpful in adjudicating the permanence of the neurologic complications. With this in mind, the longest that I have treated a patient who does not warrant life-long anticoagulation for other reasons is 2 years. For patients with headache or visual disturbances, I prescribe acetazolamide, titrating the dose up to effect. Regarding a thrombophilia evaluation, I do not feel strongly either way in this scenario and would choose to do such an evaluation in a patient with a strong family history of thrombosis but not in a patient without that.
Case 3: minimally provoked DVT with or without pulmonary embolism
Challenges and choices
As was stated earlier, the majority of VTE are due to central venous catheters and as such often occur in children with serious or chronic illnesses, or both. Thus, a relatively small percentage of cases present directly from the community, and among those, almost none are truly idiopathic, that is, some provocative factor is found either by history (oral contraceptive pill [OCP] use, trauma, local infection, repetitive use trauma) or by laboratory or imaging evaluation (inherited thrombophilia, antiphospholipid antibody syndrome [APLAS], May-Thurner anomaly).
A 16-year-old girl with a history of dysfunctional uterine bleeding who is on an OCP for management of her menorrhagia presents to an emergency room with acute onset of severe shortness of breath and is diagnosed with a right pulmonary artery embolism. In retrospect, she had had left leg pain and swelling for about 10 days but did not seek medical care. A Doppler ultrasound demonstrates a left iliofemoral DVT. She is started on UFH and transferred to the intensive care unit, and you are consulted to determine further management. The major issues for this case are as follows. (1) Is there a role for surgery in this setting? (2) Is there a role for an inferior vena cava filter? (3) Should this patient receive thrombolytic therapy? (4) What anticoagulation regimen should this patient receive? (5) Is a thrombophilia evaluation warranted? (6) How will the menorrhagia be managed in the future? (7) Should this patient be evaluated for the May-Thurner syndrome?
Although surgery can be considered in a life-threatening saddle embolus situation, it is rarely, if ever, required in pediatric PE patients. As for an inferior vena cava filter, there is no indication (especially considering that the patient already has a PE), unless there is an ongoing risk for PE, and anticoagulation is contraindicated. As for thrombolytic therapy, there are no published studies in children, and only 2 case reports have been identified.15,16 Nonetheless, this approach should always be an option in extreme and life-threatening cases. The mainstay of therapy is anticoagulation. The aforementioned ACCP guidelines5 do not specifically address the issue of PE, choosing to lump this life-threatening complication with DVT under the heading of VTE, and recommend 3 months of therapy for a provoked VTE. The choice of anticoagulant is similar to the options in case 2, with warfarin, LMWH, and fondaparinux being the primary options. An additional option in this case is to use a DOAC with the following caveats: (1) the patient has achieved full physical and sexual maturity; (2) there should be a clear rationale why the standard options are undesirable and such rationale is well documented in the medical record; (3) the overall costs (including therapeutic drug monitoring) should be similar; and (4) the patient and parents are aware that there are few published studies of DOACs that included patients of this age. With respect to a thrombophilia work-up, one must consider the pros and cons of both performing and not performing the evaluation. Although a provoking cause is present in this case, most young women who are prescribed OCPs do not develop a VTE, and the OCPs may, in this young patient, have unmasked an inherited thrombophilia or APLAS. The advantages of assessing for thrombophilia thus include identifying APLAS and an inherited thrombophilia, both of which would affect long-term management and factor in with respect to future management of her menorrhagia. The disadvantages mostly revolve around the anxiety that could be induced in the patient and family regardless of the findings. For some, a negative outcome could be distressing because it would make it more difficult to explain why this patient receiving OCPs had a life-threatening complication, whereas for others a positive outcome may invoke anxiety for the future. Thus, a careful discussion should be had once the patient is stable to determine the best course of action on an individual basis. With respect to management of the menorrhagia, the hematologist should certainly expect it to continue and possibly worsen on anticoagulation. Should the menorrhagia require management, the options are very limited, because most options aimed at minimizing the bleeding are likely to increase the risk for thrombosis, with the only clear exception being an intrauterine device (IUD).17 Lastly, this PE resulted from a left-leg DVT, thus raising the possibility of the May-Thurner syndrome and the question of whether this should be formally evaluated. The issue, however, is the specificity of the testing, because this anomaly is present in 24% of the general population,18 and a positive finding in this patient does not necessarily indicate that the DVT was a result of this condition.
How I would treat this patient
Certainly, initiating anticoagulation and other supportive measures, for example, oxygen, should be initiated immediately on diagnosis of a PE and could be considered even before objective imaging demonstrates the PE if it is clear clinically that the patient has a DVT. I would not consider surgery or thrombolysis unless the patient is in extremis and immediate resolution of the embolus is going to be life-saving. Having said that, even in several extreme cases that I have been involved in for which cardiorespiratory resuscitation was required, the only antithrombotic therapy given was anticoagulation, and the patients not only survived but made a full recovery. Conversely, children, teenagers in particular, have died of PE; thus I would not be shy to initiate thrombolysis and even consult the thoracic surgeons in life-threatening situations. I would perform a thrombophilia evaluation, including testing for APLAS, because this would affect the treatment regimen that I would prescribe. Clearly for those with APLAS, indefinite anticoagulation is indicated, and for those with “significant” inherited thrombophilia (deficiencies of proteins C or S or antithrombin deficiency), I would consider indefinite anticoagulation as well, given that this patient had a PE. For those with DVT without PE, I would discontinue anticoagulation at 3 or 6 months, depending on their follow-up imaging studies. For those with “milder” thrombophilia, such as factor V Leiden or the prothrombin mutation, I would treat them in the same way as those with a negative evaluation except for their menorrhagia management (see below). With respect to the duration of anticoagulation, one could opt for 3 months of therapy, as is recommended by the ACCP guidelines, and certainly this is a reasonable approach. In my personal experience, I have found this duration to be too short, with both recurrences of PE occurring shortly after discontinuation of anticoagulation and ongoing pulmonary symptoms such as dyspnea and cough continuing in others. Thus, my practice has been to be very conservative in such patients, continuing therapy for a period of 6 to 12 months. Although there is no evidence that extending therapy is beneficial, I believe that the low risk of continued anticoagulation outweighs the potentially life-threatening consequences of an early recurrence. Patients who are having bleeding issues with anticoagulation would be treated for 6 months, and those who tolerate anticoagulation well would be treated for 12 months. Regarding the choice of anticoagulant, I would use similar logic in this case as in the previous case, with the only exception being consideration for treatment with a DOAC. In my view, I would prefer to use a standard anticoagulant and only use a DOAC if there are extenuating circumstances; given the lack of trial results, however, a savvy patient and family may ask why their nearly adult daughter could not be treated as an adult with a DOAC. I believe it is not unreasonable to prescribe a DOAC under such circumstances with the caveats previously mentioned.
Managing menorrhagia in this patient could be very challenging, recalling that the rationale for her to be on OCPs was heavy menstrual bleeding in the first place. There simply are not ideal or simple options. Any systemic hormonal therapy, even those containing only progesterone, carry with them some risk for thrombosis.19 Furthermore, progesterone-only methods may not be as effective as are those combined with estrogen. Therefore, the only truly effective and safe option is the levonorgestrel-impregnated IUDs (Mirena and Skyla, Bayer, Whippany, NJ).20 Given the above, and if only considering management of menorrhagia, I would discuss taking either a wait-and-see approach with this patient, hoping that her dysfunctional uterine bleeding resolves, or have an adolescent medicine specialist or gynecologist insert an IUD. If, however, the patient is sexually active and requires contraception, again the best option would be an IUD; however, given that the patient will be on anticoagulation, one could consider reinstituting OCPs, especially ones with a lower estrogen content. Finally, I would request interventional radiology to evaluate for May-Thurner syndrome but only after complete clot resolution and close to the planned end of anticoagulation therapy. Despite their being normal individuals with May-Thurner who will never develop a DVT, I believe that a patient who had a life-threatening VTE, arising presumably from a left-lower-extremity DVT, should be evaluated for this anomaly.
Summary
Management of VTE in children is highly complex and highly individualized. The 3 cases presented only scratch the surface of possible scenarios that a hematologist will encounter. Perhaps the most important counsel is to seek advice from those hematologists who have dedicated their careers to the care for such patients, especially for cases that are not straightforward. Other general recommendations are as follows: (1) ensure proper communication among the (often) many consultants caring for these patients and the primary care team; (2) treat each patient individually (although it is a bit of a cliché to say this, it nevertheless is true that no 2 children with VTE are the same); (3) ensure a concerted team effort from within the hematology team (nurses, nurse practitioners, pharmacists, physical therapists, social workers) that most pediatric hematologists already work with daily; and (4) follow patients closely throughout the resolution process. In general we see patients monthly at least for the first 3 months of therapy to ensure proper adherence and assessment for adverse events. Lastly, it is important to note that while there currently is a paucity of high-level evidence in pediatric thrombosis, numerous trials are under way as a result of the DOAC revolution, and in the coming years, we will learn much as a community, and the way we manage children with VTE will likely undergo significant changes.
Acknowledgments
The author would like to acknowledge the pioneers in this field whom he had the opportunity to meet during his fellowship and who inspired him to pursue a career in pediatric thrombosis: Marilyn Manco-Johnson and the late Maureen Andrew. The author would also like to acknowledge his 2 mentors who supported and guided him throughout his career development and without whom he would never have been invited to write this paper: Naomi Luban and Diane Nugent. In addition, the author would like to give thanks to the many people who have supported his research over the past 17 years.
The author would like to acknowledge the US Food and Drug Administration (grant number 1R01FD003091) and the National Institutes of Health, National Heart, Lung, and Blood Institute (grant number 1R01HL095110), both of which supported his research in pediatric thrombosis.
Authorship
Contribution: G.Y. conceived of, wrote, and edited the manuscript.
Conflict-of-interest disclosure: G.Y. participates in the steering committees for rivaroxaban and edoxaban, for which he has received financial compensation.
Correspondence: Guy Young, Children’s Hospital Los Angeles, 4650 Sunset Blvd, Mail Stop 54, Los Angeles, CA 90027; e-mail: gyoung@chla.usc.edu.