Key Points
The MALT-IPI was built by using 401 patients in the IELSG-19 randomized trial and validated in an independent set (N = 633).
This novel disease-specific index efficiently discriminates patient with good, intermediate, and poor outcomes.
Abstract
There are no widely accepted prognostic indices for extranodal marginal zone lymphoma of mucosa-associated lymphoid tissue (MALT). This study aimed to develop and validate a specific prognostic tool to personalize and optimize treatment of patients with MALT lymphoma. A prognostic index was built by Cox regression (stepwise selection) using data from 401 patients enrolled in the international randomized International Extranodal Lymphoma Study Group 19 (IELSG-19) trial (NCT 00210353). A validation set, including 633 patients, was obtained by merging 3 independent cohorts of MALT lymphoma patients. The 3 individual features maintaining the greatest prognostic significance for event-free survival (EFS, the main endpoint of the IELSG-19 trial) were age ≥70 years (hazard ratio [HR], 1.72; 95% confidence interval [CI], 1.26-2.33), Ann Arbor stage III or IV (HR, 1.79; 95% CI ,1.35-2.38), and an elevated lactate dehydrogenase level (HR, 1.87; 95% CI, 1.27-2.77). The prognostic index (MALT-IPI) constructed using these 3 parameters identified 3 groups: low, intermediate, and high risk (corresponding to the presence of 0, 1, or ≥2 of these factors, respectively). The 5-year EFS rates in the low-, intermediate-, and high-risk groups were 70%, 56%, and 29%, respectively. The MALT-lymphoma International Prognostic Index (MALT-IPI) also significantly discriminated between patients with different progression-free, overall, and cause-specific survival. The prognostic utility was retained in gastric and nongastric lymphomas, in each treatment arm (chlorambucil, rituximab, and rituximab plus chlorambucil), and was confirmed in the validation set. The new index, MALT-IPI, is a simple, accessible, and effective tool to identify MALT lymphoma patients at risk of poor outcomes. It may help define appropriate treatment approaches for individual patients.
Medscape Continuing Medical Education online

In support of improving patient care, this activity has been planned and implemented by Medscape, LLC and the American Society of Hematology. Medscape, LLC is jointly accredited by the Accreditation Council for Continuing Medical Education (ACCME), the Accreditation Council for Pharmacy Education (ACPE), and the American Nurses Credentialing Center (ANCC), to provide continuing education for the healthcare team.
Medscape, LLC designates this Journal-based CME activity for a maximum of 1.00 AMA PRA Category 1 Credit(s)™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
All other clinicians completing this activity will be issued a certificate of participation. To participate in this journal CME activity: (1) review the learning objectives and author disclosures; (2) study the education content; (3) take the post-test with a 75% minimum passing score and complete the evaluation at http://www.medscape.org/journal/blood; and (4) view/print certificate. For CME questions, see page 1486.
Disclosures
Associate Editor Laurie H. Sehn served as an advisor or consultant for AbbVie, Amgen, Roche/Genentech, Celgene, Janssen, Lundbeck, Seattle Genetics, and TG Therapeutics. CME questions author Laurie Barclay, freelance writer and reviewer, Medscape, LLC, owns stock, stock options, or bonds from Alnylam, Biogen, and Pfizer Inc. The authors declare no competing financial interests.
Learning objectives
Compare prognostic features incorporated in the mucosa-associated lymphoid tissue lymphoma international prognostic index (MALT-IPI).
Evaluate the ability of the MALT-IPI to differentiate outcomes among low-, intermediate-, and high-risk groups of patients with MALT lymphoma.
Assess clinical implications regarding use of the MALT-IPI to identify low-, intermediate-, and high-risk groups of patients with MALT lymphoma.
Release date: September 21, 2017; Expiration date: September 21, 2018
Introduction
The International Prognostic Index (IPI) for diffuse large B-cell lymphomas was developed >2 decades ago1 and has proven to be a reliable prognostic tool for choosing treatment for these patients and guiding stratification in clinical trials. It has since been revised, taking into account the effect of more recent treatments on the natural history of the disease.2,3 Although attempts have been made to prove the usefulness of the IPI in low-grade lymphoma patients,4,5 its aptness for other lymphoma subtypes has been questioned. This led to the proposal of lymphoma-specific prognostic indices, the follicular lymphoma international prognostic index (FLIPI and FLIPI2),6,7 the mantle cell lymphoma international prognostic index (MIPI),8,9 and others for less common conditions.10,11
Marginal zone lymphomas (MZLs) represent a heterogeneous subgroup, classified as extranodal, splenic, and nodal. Extranodal cases, also termed mucosa-associated lymphoid tissue (MALT), account for ∼70% of MZLs.12 MALT lymphoma itself is a heterogeneous disease with a correspondingly variable clinical presentation.13-16 Patients are managed with a variety of treatments, which generally result in good outcomes.17-20 Nonetheless, with the exception of Helicobacter pylori eradication in localized gastric lymphoma, the selection of an appropriate treatment can be challenging and, in the absence of a consensus, a simple validated prediction tool specific to MALT lymphoma patients could offer a means to optimize therapeutic management of this rare population.
Application of the original and modified IPI or FLIPI in MZL patients has been proposed from small retrospective studies13,21,22 but they have never been properly validated. We therefore sought to develop a MALT lymphoma IPI (MALT-IPI) for patients treated during the rituximab era by developing a model using the patient data set of the largest recent controlled clinical trial in MALT lymphoma, namely the International Extranodal Lymphoma Study Group 19 (IELSG-19) randomized study, conducted by the International Extranodal Lymphoma Study Group in 6 European countries, which showed the superiority of the combination of chlorambucil and rituximab in comparison with either rituximab or chlorambucil given as single agents.23,24
Patients and methods
Study design and data collection
This study was conducted with data from the final analysis of the international IELSG-19 randomized phase III trial (NCT 00210353),24 conducted at 77 centers in 6 countries with the cooperation of major collaborative trial groups (Fondazione Italiana Linfomi, Groupe d’Etude des Lymphomes de l’Adulte, UK National Cancer Research Institute, and Grup per l’Estudi dels Limfomes de Catalunya i Balears). The study was approved by the institutional review board or ethics committee of each institution. All patients provided written informed consent. This trial compared chlorambucil alone to rituximab alone and to the combination of rituximab and chlorambucil (R-chlorambucil) as front-line therapy in MALT lymphoma patients, with event-free survival (EFS) as the primary endpoint.23,24 Patients were stratified according to tumor site (gastric vs nongastric), nodal involvement, prior local therapy, and IPI risk group.
The study database contained the information needed to assess EFS, progression-free survival (PFS), cause-specific survival (CSS), and overall survival (OS) rates together with dichotomized variables indicating patient age (<70 vs ≥70 years, a cutoff generated by receiver-operating characteristic analysis as the one with the best classification rate), Eastern Cooperative Oncology Group performance status (ECOG PS, 0-1 vs ≥2), Ann Arbor stage (I-II vs III-IV), primary disease site (gastric vs nongastric), nodal involvement (present vs absent), number of extranodal sites (<2 vs ≥2), bone marrow involvement (present vs absent), serum β2 microglobulin level (<3 mg/L vs ≥3 mg/L), serum lactate dehydrogenase (LDH) level (normal vs elevated), and B symptoms (present vs absent). Spleen and tonsils were considered as extranodal sites. Involved areas were identified by clinical examination, computed tomography scan, magnetic resonance imaging, or endoscopic investigations.
Statistical analysis
An initial exploratory analysis was performed on the patient data set (N = 393; median follow-up, 5.6 years) for a preliminary meeting communication25 to identify among the dichotomized variables those with the greatest prognostic impact on EFS, PFS, CSS, and OS. A putative prognostic model discriminating 3 risk groups was established by Cox regression on EFS (the main endpoint of the IELSG-19 study).25 This procedure included all variables identified as statistically significant by univariate analysis, namely: age, PS, serum LDH concentration, stage, gastric localization, nodal involvement, and number of extranodal sites. A definitive analysis was then performed with the use of the final study database (N = 401; median follow-up, 7.4 years) as the test set. A stepwise backward selection process with a criterion of P < .005 for variable retention was used. Serum β2 microglobulin concentration was not included in the model owing to the high rate (28%) of missing observations.
The MALT-IPI was then built, dividing patients into 3 risk groups, namely, a low-, intermediate-, and high-risk group, based on the presence of 0, 1, or ≥2 of the identified variables, respectively.
A validation set was obtained by merging 3 independent patient cohorts:
The database of the IELSG-1 study of nongastric MALT lymphomas, a retrospective survey of 180 patients, all with diagnosis confirmed by central pathology review, treated between 1983 and 1999 at 20 international institutions.13
A series of 185 MALT lymphoma patients diagnosed between 1995 and 2012 at the Oncology Institute of Southern Switzerland, (Bellinzona, Switzerland) and the Haematology Division of the Amedeo Avogadro University of Eastern Piedmont (Novara, Italy), which was partly included in a recent study of histologic transformation in marginal zone lymphomas.26
An unpublished series of 268 MALT lymphoma patients from the database of the Medical University of Vienna, diagnosed between 1999 and 2015 (provided by M. Raderer and B. Kiesewetter).
The validation set contained information on the IPI1 and the revised international prognostic index (R-IPI)2 risk group for most individual patients.
EFS, PFS, OS, and CSS, were defined according to the revised response criteria for malignant lymphomas.27 Follow-up was calculated as the median time to censoring or death by using a reverse Kaplan-Meier analysis.28 Survival probabilities were calculated by using the life-table method, and survival curves were estimated by the Kaplan-Meier method with upper and lower confidence intervals (CIs) calculated by using Greenwood’s approximation; differences between curves were analyzed by using the log-rank test.29 Associations were analyzed by using the χ2 test or the Fisher’s exact test, as appropriate. The Cox proportional hazards model30 was used for the estimation of the hazard ratio (HR) and its CIs, with the low-risk group as the reference group. Binomial exact 95% CIs were calculated for incidence percentages. Performance of prognostic indices was compared by using Gönen and Heller’s k statistic, a concordance probability estimate that calculates the probability of agreement for any pair of patients, where agreement means that the patient with the shorter survival time also has the larger risk score31 ; higher values of k indicate better discrimination. Statistical analyses were conducted by using the STATA statistical software package, version 11 (StataCorp, College Station, TX) and the R statistical software environment, version 3.1.1 (https://www.r-project.org).
Results
Elaboration of the MALT-IPI
A total of 401 patients, prospectively enrolled and treated in the IELSG-19 randomized study, were analyzed. The median follow-up time was 7.4 years. Nearly one-quarter of this population (92 patients, 22.9%) was >70 years of age. Primary gastric MALT lymphoma was diagnosed in 171 patients (42.6%), and disseminated disease was present at diagnosis in 175 (43.6%), with 123 (30.7%) having >1 extranodal site. Lymph node involvement was present in one-third of the population (142 patients, 35.4%). Elevated LDH and poor performance status were infrequent (10.5% and 1.5%, respectively). Several clinicopathological characteristics were identified with univariate analysis of EFS, PFS, CSS, and OS to predict for poor outcomes, namely age ≥70 years, ECOG PS ≥2, Ann Arbor stage III or IV, elevated LDH level, nodal and multiple involvement, and primary nongastric site (Table 1). A subsequent stepwise Cox regression was then performed (including 400 patients with no missing data) that identified 3 independent predictors of shorter EFS (Table 2) (ie, Ann Arbor stage III or IV [HR, 1.79], age ≥70 years [HR, 1.72], and elevated LDH levels [HR, 1.87]). The MALT-IPI could then be built with 3 risk groups defined by the presence of 0 (low risk, n = 167), 1 (intermediate risk, n = 165), or ≥2 (high risk, n = 68) of these three variables. The index not only distinguished patients with significantly different EFS and PFS, but also allowed the identification of patients with significantly shorter OS and CSS (Figure 1). Table 3 reports the Kaplan-Meier estimates for all the survival endpoints and Table 4 reports the HRs for the intermediate- and high-risk groups using the low-risk group as the reference group.
Univariate analysis of potential prognostic factors for EFS, PFS, CSS, and OS in the patients treated in the randomized IELSG-19 study
| Parameter . | Group (N) . | EFS . | PFS . | CSS . | OS . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5-y rate, % (95% CIs) . | Median, y . | P . | 5-y rate, % (95% CIs) . | Median, y . | P . | 5-y rate, % (95% CI) . | Median, y . | P . | 5-y rate, % (95% CI) . | Median, y . | P . | ||
| Age ≥70 y | Yes (92) | 43.1 (32.8-53.0) | 3.4 | .0002 | 67.4 (61.5-72.5) | NR | .0001 | 86.6 (77.1-92.4) | NR | <.0001 | 68.5 (57.6-77.1) | 9.1 | <.0001 |
| No (309) | 60.9 (55.1-66.1) | 9.8 | 55.8 (44.8-65.4) | 4.6 | 99.6 (97.6-99.9) | NR | 96.9 (94.1-98.4) | NR | |||||
| Male sex | Yes (197) | 54.6 (47.2-61.3) | 7.4 | .3403 | 60.4 (52.8-67.1) | 9.1 | .3074 | 96.6 (92.6-98.5) | NR | .7256 | 89.7 (84.3-93.3) | NR | .8342 |
| No (204) | 58.8 (51.7-65.3) | 9.8 | 62.2 (57.9-71.6) | NR | 96.9 (93.3-98.6) | NR | 90.1 (85.9-94.2) | NR | |||||
| B symptoms | Yes (42) | 50.7 (34.5-64.9) | 7.4 | .1922 | 55.3 (38.1-69.6) | 7.4 | .1567 | 86.5 (70.5-94.2) | NR | .0028 | 80.0 (63.9-89.5) | NR | .0125 |
| No (359) | 57.5 (52.1-62.4) | 8.6 | 63.7 (58.2-68.6) | NR | 97.9 (95.6-99.0) | NR | 91.5 (88.0-94.0) | NR | |||||
| ECOG PS ≥ 2 | Yes (6) | 16.7 (0.8-51.7) | 0.8 | .0277 | 16.7 (0.7-51.7) | 1.4 | .0046 | 80.0 (20.4-96.9) | 5.4 | <.0001 | 66.7 (19.5-94.4) | 5.3 | <.0001 |
| No (395) | 57.3 (52.2-62.1) | 8.6 | 63.6 (58.4-68.4) | NR | 97.0 (94.7-98.3) | NR | 90.7 (87.2-93.2) | NR | |||||
| β2MG >UNL | Yes (46) | 43.2 (28.7-56.9) | 3.9 | .0315 | 48.0 (32.3-62.0) | 4.9 | .0296 | 90.8 (77.2-96.4) | NR | .0084 | 82.5 (68.1-90.8) | NR | .0010 |
| No (243) | 60.7 (54.2-66.6) | 9.6 | 66.6 (60.0-72.4) | NR | 98.3 (95.5-99.3) | NR | 93.5 (89.5-96.0) | NR | |||||
| LDH >UNL | Yes (42) | 35.6 (21.6-49.9) | 1.9 | .0004 | 41.2 (25.8-56.0) | 2.4 | .0001 | 92.3 (78.0-97.5) | NR | .0071 | 80.3 (64.4-89.6) | NR | .0087 |
| No (358) | 59.4 (54.0-64.4) | 9.6 | 65.6 (60.2-70.5) | NR | 97.3 (94.9-98.6) | NR | 91.7 (88.3-94.2) | NR | |||||
| Stage III-IV | Yes (175) | 44.2 (36.7-51.5) | 3.5 | <.0001 | 49.6 (41.5-57.1) | 4.9 | <.0001 | 94.4 (89.4-97.0) | NR | .0070 | 84.5 (78.0-89.2) | NR | .0027 |
| No (226) | 66.5 (59.8-72.3) | NR | 73.2 (66.6-78.7) | NR | 98.6 (95.7-99.5) | NR | 94.8 (90.9-97.1) | NR | |||||
| Gastric localization | Yes (171) | 62.3 (54.4-69.2) | 10.1 | .0241 | 70.8 (62.9-77.3) | NR | .0013 | 98.1 (94.3-99.4) | NR | .198 | 93.1 (87.9-96.1) | NR | .0729 |
| No (230) | 52.6 (45.9-58.9) | 5.7 | 57.1 (50.1-63.5) | 7.0 | 95.8 (92.1-97.8) | NR | 88.3 (83.3-91.9) | NR | |||||
| Nodal involvement | Yes (142) | 45.7 (37.2-53.8) | 3.4 | .0001 | 51.2 (42.1-59.5) | 5.6 | <.0001 | 95.2 (89.7-97.8) | NR | .0465 | 85.1 (77.8-90.1) | NR | .0385 |
| No (259) | 62.7 (56.4-68.3) | NR | 69.2 (62.8-74.6) | NR | 97.6 (94.7-98.9) | NR | 93.1 (89.2-95.7) | NR | |||||
| Extranodal sites >1 | Yes (123) | 48.5 (39.3-57.1) | 4.3 | .0157 | 52.3 (42.8-61.0) | 5.7 | .0022 | 93.7 (87.3-97.0) | NR | .0474 | 83.7 (75.6-89.3) | NR | .0035 |
| No (278) | 60.3 (54.2-65.9) | 9.6 | 67.7 (61.5-73.1) | NR | 98.1 (95.4-99.2) | NR | 93.2 (89.4-95.7) | NR | |||||
| Parameter . | Group (N) . | EFS . | PFS . | CSS . | OS . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5-y rate, % (95% CIs) . | Median, y . | P . | 5-y rate, % (95% CIs) . | Median, y . | P . | 5-y rate, % (95% CI) . | Median, y . | P . | 5-y rate, % (95% CI) . | Median, y . | P . | ||
| Age ≥70 y | Yes (92) | 43.1 (32.8-53.0) | 3.4 | .0002 | 67.4 (61.5-72.5) | NR | .0001 | 86.6 (77.1-92.4) | NR | <.0001 | 68.5 (57.6-77.1) | 9.1 | <.0001 |
| No (309) | 60.9 (55.1-66.1) | 9.8 | 55.8 (44.8-65.4) | 4.6 | 99.6 (97.6-99.9) | NR | 96.9 (94.1-98.4) | NR | |||||
| Male sex | Yes (197) | 54.6 (47.2-61.3) | 7.4 | .3403 | 60.4 (52.8-67.1) | 9.1 | .3074 | 96.6 (92.6-98.5) | NR | .7256 | 89.7 (84.3-93.3) | NR | .8342 |
| No (204) | 58.8 (51.7-65.3) | 9.8 | 62.2 (57.9-71.6) | NR | 96.9 (93.3-98.6) | NR | 90.1 (85.9-94.2) | NR | |||||
| B symptoms | Yes (42) | 50.7 (34.5-64.9) | 7.4 | .1922 | 55.3 (38.1-69.6) | 7.4 | .1567 | 86.5 (70.5-94.2) | NR | .0028 | 80.0 (63.9-89.5) | NR | .0125 |
| No (359) | 57.5 (52.1-62.4) | 8.6 | 63.7 (58.2-68.6) | NR | 97.9 (95.6-99.0) | NR | 91.5 (88.0-94.0) | NR | |||||
| ECOG PS ≥ 2 | Yes (6) | 16.7 (0.8-51.7) | 0.8 | .0277 | 16.7 (0.7-51.7) | 1.4 | .0046 | 80.0 (20.4-96.9) | 5.4 | <.0001 | 66.7 (19.5-94.4) | 5.3 | <.0001 |
| No (395) | 57.3 (52.2-62.1) | 8.6 | 63.6 (58.4-68.4) | NR | 97.0 (94.7-98.3) | NR | 90.7 (87.2-93.2) | NR | |||||
| β2MG >UNL | Yes (46) | 43.2 (28.7-56.9) | 3.9 | .0315 | 48.0 (32.3-62.0) | 4.9 | .0296 | 90.8 (77.2-96.4) | NR | .0084 | 82.5 (68.1-90.8) | NR | .0010 |
| No (243) | 60.7 (54.2-66.6) | 9.6 | 66.6 (60.0-72.4) | NR | 98.3 (95.5-99.3) | NR | 93.5 (89.5-96.0) | NR | |||||
| LDH >UNL | Yes (42) | 35.6 (21.6-49.9) | 1.9 | .0004 | 41.2 (25.8-56.0) | 2.4 | .0001 | 92.3 (78.0-97.5) | NR | .0071 | 80.3 (64.4-89.6) | NR | .0087 |
| No (358) | 59.4 (54.0-64.4) | 9.6 | 65.6 (60.2-70.5) | NR | 97.3 (94.9-98.6) | NR | 91.7 (88.3-94.2) | NR | |||||
| Stage III-IV | Yes (175) | 44.2 (36.7-51.5) | 3.5 | <.0001 | 49.6 (41.5-57.1) | 4.9 | <.0001 | 94.4 (89.4-97.0) | NR | .0070 | 84.5 (78.0-89.2) | NR | .0027 |
| No (226) | 66.5 (59.8-72.3) | NR | 73.2 (66.6-78.7) | NR | 98.6 (95.7-99.5) | NR | 94.8 (90.9-97.1) | NR | |||||
| Gastric localization | Yes (171) | 62.3 (54.4-69.2) | 10.1 | .0241 | 70.8 (62.9-77.3) | NR | .0013 | 98.1 (94.3-99.4) | NR | .198 | 93.1 (87.9-96.1) | NR | .0729 |
| No (230) | 52.6 (45.9-58.9) | 5.7 | 57.1 (50.1-63.5) | 7.0 | 95.8 (92.1-97.8) | NR | 88.3 (83.3-91.9) | NR | |||||
| Nodal involvement | Yes (142) | 45.7 (37.2-53.8) | 3.4 | .0001 | 51.2 (42.1-59.5) | 5.6 | <.0001 | 95.2 (89.7-97.8) | NR | .0465 | 85.1 (77.8-90.1) | NR | .0385 |
| No (259) | 62.7 (56.4-68.3) | NR | 69.2 (62.8-74.6) | NR | 97.6 (94.7-98.9) | NR | 93.1 (89.2-95.7) | NR | |||||
| Extranodal sites >1 | Yes (123) | 48.5 (39.3-57.1) | 4.3 | .0157 | 52.3 (42.8-61.0) | 5.7 | .0022 | 93.7 (87.3-97.0) | NR | .0474 | 83.7 (75.6-89.3) | NR | .0035 |
| No (278) | 60.3 (54.2-65.9) | 9.6 | 67.7 (61.5-73.1) | NR | 98.1 (95.4-99.2) | NR | 93.2 (89.4-95.7) | NR | |||||
β2-MG, serum β2 microglobulin; NR, not reached; UNL, upper normal limit.
Final model for EFS generated by stepwise Cox regression used to build the MALT-IPI
| N = 400 . | HR . | Standard Error . | 95% CI . | P . |
|---|---|---|---|---|
| Stage III-IV | 1.79 | 0.26 | 1.35-2.38 | <.001 |
| Age >70 y | 1.72 | 0.27 | 1.26-2.33 | .001 |
| LDH >UNL | 1.87 | 0.37 | 1.27-2.77 | .002 |
| N = 400 . | HR . | Standard Error . | 95% CI . | P . |
|---|---|---|---|---|
| Stage III-IV | 1.79 | 0.26 | 1.35-2.38 | <.001 |
| Age >70 y | 1.72 | 0.27 | 1.26-2.33 | .001 |
| LDH >UNL | 1.87 | 0.37 | 1.27-2.77 | .002 |
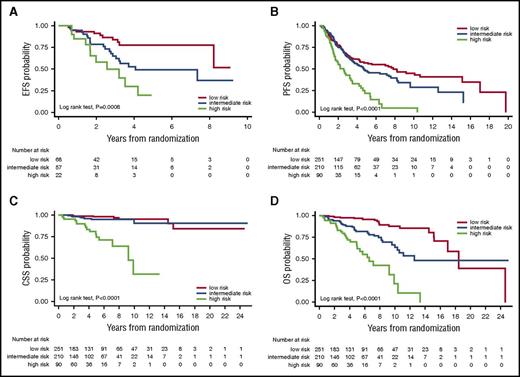
Clinical outcomes according to MALT-IPI risk groups in the testing set. Kaplan-Meier estimates for EFS (A), PFS (B), CSS (C), and OS (D) in the testing set from the IELSG-19 clinical trial database.
Clinical outcomes according to MALT-IPI risk groups in the testing set. Kaplan-Meier estimates for EFS (A), PFS (B), CSS (C), and OS (D) in the testing set from the IELSG-19 clinical trial database.
Analysis of survival endpoints (EFS, PFS, CSS, and OS) according to the MALT-IPI risk group in the testing set from the IELSG-19 clinical trial database: Kaplan-Meier estimates
| Risk group . | N . | EFS . | PFS . | CSS . | OS . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5-y rate, % (95% CI) . | Median, y . | P . | 5-y rate, % (95% CI) . | Median, y . | P . | 5-y rate, % (95% CI) . | Median, y . | P . | 5-y rate, % (95% CI) . | Median, y . | P . | ||
| Low | 167 | 69.8 (62.0-76.2) | NR | <.0001 | 76.0 (68.3-82.1) | NR | <.0001 | 100 (–) | NR | <.0001 | 98.7 (94.9-99.7) | NR | <.0001 |
| Intermediate | 165 | 55.7 (47.7-63.0) | 6.3 | 63.1 (54.8-70.2) | 9.8 | 98.1 (94.1-99.4) | NR | 93.1 (87.8-96.1) | NR | ||||
| High | 68 | 28.7 (18.4-39.9) | 2.0 | 32.5 (21.2-44.2) | 2.8 | 84.5 (72.3-91.7) | NR | 64.3 (51.3-74.8) | 8.1 | ||||
| Risk group . | N . | EFS . | PFS . | CSS . | OS . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5-y rate, % (95% CI) . | Median, y . | P . | 5-y rate, % (95% CI) . | Median, y . | P . | 5-y rate, % (95% CI) . | Median, y . | P . | 5-y rate, % (95% CI) . | Median, y . | P . | ||
| Low | 167 | 69.8 (62.0-76.2) | NR | <.0001 | 76.0 (68.3-82.1) | NR | <.0001 | 100 (–) | NR | <.0001 | 98.7 (94.9-99.7) | NR | <.0001 |
| Intermediate | 165 | 55.7 (47.7-63.0) | 6.3 | 63.1 (54.8-70.2) | 9.8 | 98.1 (94.1-99.4) | NR | 93.1 (87.8-96.1) | NR | ||||
| High | 68 | 28.7 (18.4-39.9) | 2.0 | 32.5 (21.2-44.2) | 2.8 | 84.5 (72.3-91.7) | NR | 64.3 (51.3-74.8) | 8.1 | ||||
Analysis of survival endpoints (EFS, PFS, CSS, and OS) according to the MALT-IPI risk group in the testing set from the IELSG-19 clinical trial database: risk estimate in Cox models with the low-risk group as the reference group
| Risk Group . | N . | EFS . | PFS . | CSS . | OS . |
|---|---|---|---|---|---|
| HR (95% CI) . | HR (95% CI) . | HR (95% CI) . | HR (95% CI) . | ||
| Low | 167 | 1 | 1 | 1 | 1 |
| Intermediate | 165 | 1.5 (1.1-2.1) | 1.7 (1.2-2.5) | 5.3 (0.6-45.6) | 2.4 (1.05-5.5) |
| High | 68 | 3.3 (2.3-4.8) | 3.9 (2.6-5.9) | 41.5 (5.4-319.7) | 13.7 (6.3-29.9) |
| Risk Group . | N . | EFS . | PFS . | CSS . | OS . |
|---|---|---|---|---|---|
| HR (95% CI) . | HR (95% CI) . | HR (95% CI) . | HR (95% CI) . | ||
| Low | 167 | 1 | 1 | 1 | 1 |
| Intermediate | 165 | 1.5 (1.1-2.1) | 1.7 (1.2-2.5) | 5.3 (0.6-45.6) | 2.4 (1.05-5.5) |
| High | 68 | 3.3 (2.3-4.8) | 3.9 (2.6-5.9) | 41.5 (5.4-319.7) | 13.7 (6.3-29.9) |
Evaluation of the prognostic index model in main clinical subgroups
The MALT-IPI was examined in various subpopulations within the testing set, specifically gastric and nongastric patients, and each of the treatment arms. The frequency of low-risk patients was significantly higher (52% vs 34%, χ2 test, P = .001), and, conversely, the frequency of high-risk patients was lower (12% vs 21%) among those with primary gastric lymphoma. Nevertheless, the model (N = 400) retained its efficiency in discriminating patients with poorer prognosis in both the gastric (N = 171) and the nongastric patient (N = 229) subgroups. (supplemental Table 1, available on the Blood Web site). Likewise, the new index also maintained statistical significance in each treatment arm (chlorambucil, n = 130; rituximab, n = 138; R-chlorambucil, n = 132) for all 4 survival endpoints (supplemental Table 2).
Histologic transformation during the trial was reported in 10 patients.24 Notably, 7 out of 10 patients with histological transformation were in the high-risk group, 3 were in the intermediate-risk group, and none were in the low-risk group (Fisher exact test, P ≤ .001). Nevertheless, the ability of MALT-IPI to identify different prognostic groups for each survival endpoint remained independent of histological transformation (Cox regression, data not shown).
External validation of the prognostic index model
The prognostic model was then examined in a large validation set (N = 633) obtained by the unification of 3 independent MALT lymphoma cohorts, the IELSG-01 cohort, the Bellinzona-Novara cohort, and the Medical University of Vienna cohort. These cohorts differed with respect to median follow-up, patient characteristics, and clinical outcomes (supplemental Table 3). EFS estimation was possible only in patients from the IELSG-01 cohort, which, however, included only nongastric primary sites. Patients from all cohorts (with 3 missing values) contributed to PFS, CSS, and OS estimations. Overall, the patients in the validation set had significantly worse outcomes in comparison with those in the training set (supplemental Table 3). Tables 5 and 6 summarizes the analysis of all the survival endpoints in the validation set. The MALT-IPI maintained its capacity to significantly discriminate different prognostic groups with respect to each survival endpoint (Figure 2), and this held true also after controlling (by Cox regression, data not shown) for the cohort effect on outcome.
Analysis of survival endpoints (EFS, PFS, CSS and OS) according to the MALT-IPI risk group in the external validation set: Kaplan-Meier estimate
| Risk group . | N . | EFS . | PFS . | CSS . | OS . | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5-y rate, % (95% CI) . | Median, y . | P . | N . | 5-y rate, % (95% CI) . | Median, y . | P . | N . | 5-y rate, % (95% CI) . | Median, y . | P . | N . | 5-y rate, % (95% CI) . | Median, y . | P . | ||
| Low | 68 | 76 (59.4-87.3) | NR | .0008 | 251 | 56.8 (49.0-63.9) | 7.8 | <.0001 | 251 | 98.2 (94.2-99.4) | NR | <.0001 | 251 | 96.7 (92.6-98.5) | 18.4 | <.0001 |
| Intermediate | 57 | 48.4 (30.7-64.0) | 4.1 | 210 | 48.0 (39.4-56.2) | 4.7 | 210 | 94.7 (89.0-97.5) | 25.1 | 210 | 81.7 (74.1-87.3) | 12.6 | ||||
| High | 22 | 15.7 (1.1-46.4) | 2.6 | 90 | 22.7 (12.1-35.4) | 2.6 | 90 | 74.3 (58.8-84.7) | 9.2 | 90 | 64.9 (50.7-75.9) | 6.6 | ||||
| Risk group . | N . | EFS . | PFS . | CSS . | OS . | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5-y rate, % (95% CI) . | Median, y . | P . | N . | 5-y rate, % (95% CI) . | Median, y . | P . | N . | 5-y rate, % (95% CI) . | Median, y . | P . | N . | 5-y rate, % (95% CI) . | Median, y . | P . | ||
| Low | 68 | 76 (59.4-87.3) | NR | .0008 | 251 | 56.8 (49.0-63.9) | 7.8 | <.0001 | 251 | 98.2 (94.2-99.4) | NR | <.0001 | 251 | 96.7 (92.6-98.5) | 18.4 | <.0001 |
| Intermediate | 57 | 48.4 (30.7-64.0) | 4.1 | 210 | 48.0 (39.4-56.2) | 4.7 | 210 | 94.7 (89.0-97.5) | 25.1 | 210 | 81.7 (74.1-87.3) | 12.6 | ||||
| High | 22 | 15.7 (1.1-46.4) | 2.6 | 90 | 22.7 (12.1-35.4) | 2.6 | 90 | 74.3 (58.8-84.7) | 9.2 | 90 | 64.9 (50.7-75.9) | 6.6 | ||||
Analysis of survival endpoints (EFS, PFS, CSS, and OS) according to the MALT-IPI risk group in the external validation set: risk estimate in Cox models with the low-risk group as the reference group
| Risk group . | EFS . | PFS . | CSS . | OS . |
|---|---|---|---|---|
| HR (95% CI) . | HR (95% CI) . | HR (95% CI) . | HR (95% CI) . | |
| Low | 1 | 1 | 1 | 1 |
| Intermediate | 2.4 (1.1-4.9) | 1.2 (0.9-1.7) | 1.8 (0.6-5.0) | 3.1 (1.8-5.4) |
| High | 4.6 (2.0-10.9) | 2.6 (1.8-3.7) | 13.5 (5.2-34.6) | 9.3 (5.2-16.9) |
| Risk group . | EFS . | PFS . | CSS . | OS . |
|---|---|---|---|---|
| HR (95% CI) . | HR (95% CI) . | HR (95% CI) . | HR (95% CI) . | |
| Low | 1 | 1 | 1 | 1 |
| Intermediate | 2.4 (1.1-4.9) | 1.2 (0.9-1.7) | 1.8 (0.6-5.0) | 3.1 (1.8-5.4) |
| High | 4.6 (2.0-10.9) | 2.6 (1.8-3.7) | 13.5 (5.2-34.6) | 9.3 (5.2-16.9) |
Clinical outcomes according to MALT-IPI risk groups in the validation set. Kaplan-Meier estimates for EFS (A), PFS (B), CSS (C), and OS (D) in the external validation set.
Clinical outcomes according to MALT-IPI risk groups in the validation set. Kaplan-Meier estimates for EFS (A), PFS (B), CSS (C), and OS (D) in the external validation set.
Comparison of the MALT-IPI with existing prognostic indices
In the validation set (N = 633), a definite risk group allocation was possible in 551 patients (87%) according to the MALT- IPI, in 563 (90%) according to the IPI, and in 564 (90%) according to the R-IPI, respectively. The prognostic power of MALT-IPI was then compared with IPI and R-IPI by a concordance probability estimate procedure based on the Gönen and Heller k statistic.31 The performance of all models was very similar: MALT-IPI was slightly superior (highest k value) in the analysis of all survival endpoints, however, the standard errors indicate overlapping CIs (supplemental Table 4).
Discussion
Treatment of populations with rare cancers, frequently in the absence of consensus or definitive recommendations, is a major challenge for clinicians, and the availability of tools to refine therapeutic strategies is a welcome resource. Following on from the publication of the IPI and establishment of the widely accepted FLIPI and MIPI, we sought to extend the existing repertoire with the MALT-IPI. This new index is specific for MALT lymphoma patients and was generated from a large cohort of 401 patients recently diagnosed and prospectively enrolled in the IELSG-19 randomized trial.24,25 The natural history of MALT lymphoma patients has not changed since the completion of this trial (performed between 2003 and 2005), with few changes to available therapeutic options and no other published randomized phase III trials.
The MALT-IPI, built on the basis of the IELSG-19 trial main endpoint (EFS), efficiently discriminates patients with good, intermediate, and poor EFS and proved to be valuable also with respect to PFS, CSS, and OS. It was validated in a large, external series of >600 MALT lymphoma patients and appeared at least as good as the IPI, which was initially developed for diffuse large B-cell lymphoma and was later shown to be useful for predicting MALT lymphoma prognosis as well.13 As with most other published prognostic indices,2,6,8 3 distinct risk groups were identified as strongly predicting outcome in terms of the 4 survival endpoints, EFS, PFS, OS, and CSS. The distribution of IELSG-19 patients according to the 3 risk groups, corresponding to 42% (low), 41% (intermediate), and 17% (high) of the evaluated population, is similar to those reported for FLIPI and MIPI.5,7,8 Five-year OS rates were 99%, 93%, and 64%, respectively, showing the greatest discrimination for the high-risk group.
Other authors have recently proposed prognostic indices for MALT lymphomas. A Korean study retrospectively evaluated 205 nongastric MZL patients treated in one institution between 1999 and 2005, with a median follow up of 3.4 years.32 They reported a prognostic index for this population in which nodal MZL, ECOG PS ≥2, and advanced stage (III/IV) were associated with poor PFS and OS, and their index proved more reliable than IPI and FLIPI for predicting OS. However, this study was limited to nongastric MZL in a Korean population, and its applicability to the wider MALT population is unclear. In 2010, Mazloom et al33 published a retrospective study using an institutional database analyzing 275 patients diagnosed with MZL between 1996 and 2003, the majority of whom (77%) had extranodal disease. Patients received a range of treatment modalities, and median follow-up was 3.9 years. Neither the IPI nor FLIPI was found to be significantly associated with outcome when applied to this latter population.33 The authors identified elevated serum β2-microglobulin levels, male sex, and the presence of B symptoms as prognostic for recurrence-free survival and OS in the extranodal MZL subgroup and constructed a prognostic index with low (32% of patients; 0 factors), intermediate (52%; 1 factor), and high (15%; ≥2 factors) risk.33 Interestingly, although the prognostic effect of β2-microglobulin levels and B symptoms (these latter only on CSS and OS) was also identified in our analysis, none of the 3 strongest prognostic factors in our analysis was captured in theirs.33 This may be due to the heterogeneous patient characteristics in the different series. Neither of these 2 MALT lymphoma prognostic models32,33 gained wide application, possibly because of their lack of validation in external cohorts.
Older age and elevated LDH levels are common to all of the lymphoma prognostic indexes, however, other variables differ, although ECOG PS ≥2, which emerged as a prognostic factor for both the modified IPI and MIPI, was not included in the FLIPI evaluation.6 Elevated LDH levels and poor ECOG PS have been correlated with shorter survival in MALT lymphomas.34,35 Several retrospective series indicated that lymph node involvement is an important prognostic factor, and some have also suggested that the concomitant involvement of multiple mucosal sites, despite being relatively common, does not necessarily carry an unfavorable impact on outcome.13,16,36,37
In the original IPI analysis, age was dichotomized at 60 years because this represented the eligibility cut point for myeloablative therapy and autologous stem cell transplant, which was restricted to younger patients. Today, this limit no longer holds true. In our index, we included an age cutoff of 70 years, defined in our study population by receiver-operating characteristic analysis as the one with the best classification rate. This cut point was shown to be a highly significant predictor of outcome. Interestingly, other studies have also described age >70 years to be associated with greater myelosuppression,38 increased comorbidities, and poorer outcome, particularly in diffuse large B-cell lymphoma.39,40 This cut point seems likely to better delineate the effect of age as a risk factor in the current era of growth factors and rituximab, which is also characterized by an increasing number of fit elderly patients. In this context, it is interesting that a large Surveillance, Epidemiology, and End Results study with >9800 patients showed that age was significant as a prognostic factor in a MALT lymphoma–specific hazard model.41 This study also found B symptoms, male sex, and stage to affect the prognosis of patients with MALT lymphoma.41
The IPI, FLIPI, and MIPI indexes (both original and revised) all involve 4 or 5 prognostic variables to define the 3 risk (or 4) groups. Some of the variables contributing to these indices may have little relevance in MALT lymphomas, such as the white blood cell count (MIPI8 ), the number of extranodal sites (IPI1 ), or the number of nodal areas (FLIPI6 ). MALT lymphoma is by definition extranodal and early dissemination to multiple nodal areas is rare, as is leukemic presentation, although, as already mentioned, dissemination to different mucosal sites does not necessarily affect survival. This may help explain why IPI and FLIPI have not always proven prognostic for patients with MALT lymphoma.33 For the MALT-IPI, a deliberate choice was made to limit the number of prognostic factors used to build the index to the individually most powerful ones, by setting the criterion for variable retention in the stepwise Cox regression at P < .005. The aim was to increase simplicity of use while maintaining accuracy. Indeed, the MALT-IPI model needs only 3 variables (patient age, LDH level, and Ann Arbor stage), each of them readily accessible in the clinical setting. Nevertheless, the MALT-IPI showed good power to discriminate different risk groups with respect to all the relevant survival endpoints. This holds true not only in the entire study population, but also in the 2 main clinical subgroups (gastric and nongastric primary presentation) and in each treatment arm of the IELSG-19 study (chlorambucil, rituximab, and R-chlorambucil).
A large external validation set generated from 3 independent patient cohorts substantiated the MALT-IPI’s prognostic ability, showing that the discriminating capacity is retained in a large heterogeneous population of patients, regardless of baseline characteristics and treatment type. Even though outcomes in the validation cohort were worse than in IELSG-19, the MALT-IPI still identified 3 distinct prognostic groups. Cox regression controlling for the cohort effect further confirmed the validity of the prognostic model. Because the prognostic capacity of the MALT-IPI appears to be independent of treatment, it may be expected that this will remain the case for patients treated with the increasingly popular combination of rituximab and bendamustine.42
Retrospective studies have suggested that different initial treatment modalities (chemotherapy and surgery alone or combined, as well as radiotherapy) do not significantly affect survival in MALT lymphomas.13,16,36 In this context, treatment is often chosen according to the personal preference and experience of the treating physician, which may be unreliable. A MALT lymphoma–specific prognostic index may offer the potential to define the patients in whom treatment is required as well those to whom novel therapies may be offered. Besides helping to guide the choice of the appropriate therapeutic approach, a specific prognostic index can be exploited for other purposes, such as patient stratification in future prospective clinical trials. Indeed, patients with low or intermediate risk may be successfully managed with nonaggressive treatment approaches, although there is an unmet need to improve the outcome in the high-risk group, which is very efficiently identified by the novel MALT-IPI. The finding that patients with high-risk MALT-IPI score had a higher incidence of histological transformation suggests that earlier (or more aggressive) treatment may be justified in the high-risk subgroup. In contrast, nonaggressive approaches appear justified in the low risk patients.
Despite the favorable long-term outcome of MALT lymphoma overall, the subgroup with high-risk MALT-IPI shows rapid treatment failure (median PFS, 2.8 years) with current approaches, particularly if treated with single alkylating agents or with rituximab alone. Future clinical trials should be aimed at minimizing exposure to treatments with a low probability of long-term disease control in this subset of patients and at exploring the activity of promising novel targeted agents.
In conclusion, the MALT-IPI, based on age ≥70 years, elevated LDH levels, and Ann Arbor stage III or IV and generated by using the data set of the largest randomized study ever conducted in MALT lymphoma, was validated in a large, external patient cohort. This novel index promises to be a simple and powerful tool to identify patients at increased risk of progression or death, allowing treatment approaches to be modulated accordingly.
Presented in part at the 13th International Conference on Malignant Lymphoma, Lugano, Switzerland, 17- 20 June 2015.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank the IELSG-19 study investigators, data managers, and nursing staff. The authors also thank Ayda Lüönd and Rita Gianascio Gianocca for the excellent secretarial assistance.
This work was partly supported by a grant from Oncosuisse (ICP OCS-01356-03-2003): The IELSG-19 clinical trial was supported in part by an unrestricted research grant from Roche International, Ltd.
The funders had no role in study design, data collection, analysis, and interpretation, or writing of the report.
Authorship
Contribution: C.T., L.C., A.C., V.T., and E.Z. contributed to the literature search, study design, statistical analysis, and data interpretation, as well as the writing and approval of the manuscript; B.K., M.R., and G.G. provided the data set for external validation and contributed to the writing and approval of the manuscript; F.C., M.M., D.L., B.C., A.L.G., and P.W.J. contributed to data interpretation and the writing and approval of the manuscript; and E.Z. had full access to all the data in the study and had final responsibility for the decision to submit.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Emanuele Zucca, Lymphoma Unit and International Extranodal Lymphoma Study Group Operation Office, Oncology Institute of Southern Switzerland, Ospedale San Giovanni, via Ospedale, 6500 Bellinzona, Switzerland; e-mail: emanuele.zucca@eoc.ch; and Catherine Thieblemont, Hôpital Saint-Louis, Service d’Hémato-oncologie, 1 Ave Claude Vellefaux, 75010 Paris, France; e-mail: catherine.thieblemont@aphp.fr.
References
Author notes
P.W.J. and E.Z. are joint senior authors.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal