Abstract
Blood or marrow transplantation (BMT) is used with curative intent for hematologic malignancies. Conditional on surviving the first 2 years after BMT, 5-year survival generally exceeds 70%. However, the cumulative therapeutic exposures lead to premature onset of chronic health conditions, such that the 15-year cumulative incidence of severe or life-threatening chronic health conditions exceeds 40%, resulting in premature mortality. The high burden of morbidity, coupled with a long latency between BMT and the development of chronic health conditions necessitates life-long risk-based monitoring of the BMT survivors. The issues of how and when to screen BMT survivors for therapy-related complications and exacerbation of preexisting conditions are important and largely unanswered questions. For BMT survivors, screening recommendations must incorporate risks associated with pre-BMT therapy as well as risks related to transplant conditioning and graft-versus-host disease. Here, we describe our approach to monitoring BMT survivors for risk-based screening and early detection of key late-occurring or long-term complications using patient scenarios to illustrate our discussion.
Introduction
Blood or marrow transplantation (BMT) is used with curative intent for hematologic malignancies. BMT recipients are exposed to chemotherapy and/or radiation before BMT (for management of primary cancer), at BMT (for the transplant procedure), and after BMT (for graft-versus-host disease [GVHD] management and/or relapse of primary cancer). The cumulative therapeutic exposures injure normal tissues, leading to premature onset of chronic health conditions such as subsequent neoplasms (SNs),1-3 congestive heart failure (CHF),4-6 coronary artery disease,7 and musculoskeletal abnormalities.8,9 In fact, the 15-year cumulative incidence of severe or life-threatening chronic health conditions exceeds 40%,10,11 resulting in premature mortality12-17 (Figure 1). Because BMT survivors carry an inordinately high burden of morbidity and because many long-term complications may not manifest for years or even decades after BMT, survivors are in need of ongoing, life-long monitoring.
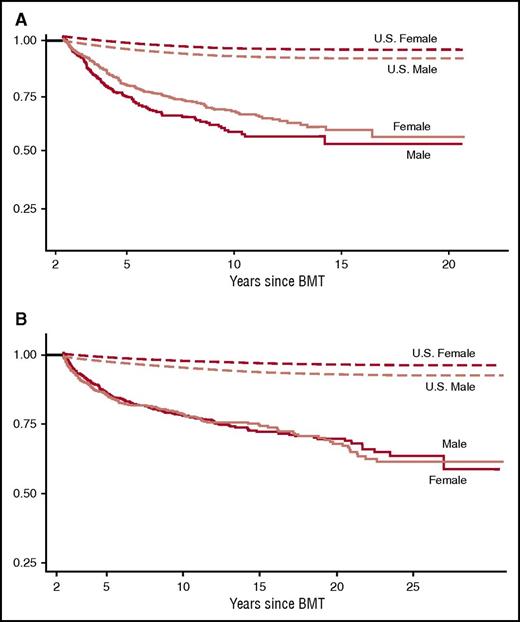
Late mortality after BMT. (A) Late mortality in 854 individuals who had survived 2 or more years after autologous BMT for hematological malignancies. Median age at BMT was 36.5 years and the median length of follow-up was 7.6 years. Overall survival was 68.8% at 10 years. Adapted from Bhatia et al.12 (B) Late mortality in 1479 individuals who had survived 2 or more years after allogeneic BMT. Median age at BMT was 25.9 years and median length of follow-up was 9.5 years. Conditional survival probability at 15 years was 80.2 years for those who remained disease-free at entry into the cohort. Adapted from Bhatia et al.13
Late mortality after BMT. (A) Late mortality in 854 individuals who had survived 2 or more years after autologous BMT for hematological malignancies. Median age at BMT was 36.5 years and the median length of follow-up was 7.6 years. Overall survival was 68.8% at 10 years. Adapted from Bhatia et al.12 (B) Late mortality in 1479 individuals who had survived 2 or more years after allogeneic BMT. Median age at BMT was 25.9 years and median length of follow-up was 9.5 years. Conditional survival probability at 15 years was 80.2 years for those who remained disease-free at entry into the cohort. Adapted from Bhatia et al.13
The issue of how and when to screen BMT survivors for complications is important, yet largely unaddressed. Recommendations for screening for BMT survivors have been developed jointly by the European Group for Blood and Marrow Transplantation, the Center for International Blood and Marrow Transplant Research, and the American Society of Blood and Marrow Transplant.18 These recommendations are focused on risks faced by patients who have survived 6 or more months after transplantation. Most of these recommendations are derived from studies that have identified specific complications (and associated risk factors) in survivors. For pediatric survivors, the Children’s Oncology Group (COG) has developed long-term follow-up guidelines (www.survivorshipguidelines.org).19 COG guidelines are evidence-based (using established associations between therapeutic exposures and late effects to identify high-risk populations) and consensus-based (matching the magnitude of risk of complications with intensity of screening). Thus, for both the pediatric and adult BMT survivor population, we do not have evidence to support the frequency, modality, or timing of screening. Instead, we rely upon consensus arrived by experts in the field. This is because small samples and/or long latency of complications prevent us from conducting randomized clinical trials to determine what intensity/modality would be superior in early detection of complications or reduction in mortality.
Here, we describe our approach to risk-based screening for late-occurring or long-term complications in BMT survivors, using patient scenarios to illustrate our discussion. Because treatment exposures vary by primary diagnosis, BMT type (autologous or allogeneic) and patient age, a therapy-based approach was adopted (Table 1). We focus primarily on patients who have undergone BMT as adults, because a recent publication described recommendations for screening patients transplanted during childhood.20
Patient 1
The patient is a 52-year-old male with a history of nodular sclerosing Hodgkin lymphoma (HL, stage IVA) diagnosed at age 34, treated with Adriamycin [doxorubicin], 300 mg/m2; bleomycin,120 units/m2; vinblastine, 72 mg/m2; and dacarbazine, 4.5 g/m2 chemotherapy, followed by involved field radiation to the mediastinum (3600 cGy). His disease relapsed 6 months after completion of therapy and he received salvage chemotherapy with ifosfamide (18 g/m2), carboplatin (1.6 g/m2), and etoposide (1 g/m2), followed by stem cell mobilization with granulocyte colony-stimulating factor, and autologous BMT with BiCNU (carmustine), 300 mg/m2; etoposide, 800 mg/m2; Ara-C (cytarabine), 1.6 g/m2; and melphalan, 140 mg/m2 conditioning. He tolerated the treatment well and was discharged home after a relatively uneventful course in the hospital. His medical care was transitioned to his primary care physician (PCP) 5 years after BMT. At age 48, he developed type 2 diabetes, managed with metformin and diet. At age 50, he developed prehypertension (systolic blood pressure [BP], 130 mm Hg; diastolic BP, 80 mm Hg), but was not treated. He now presents to his PCP with a 6-month history of worsening fatigue, shortness of breath, and lower extremity swelling. His clinical examination is notable for rales over the lung bases, and 1+ pitting pedal edema extending up to his ankles. Chest radiograph is concerning for cardiomegaly and pulmonary interstitial edema. Echocardiogram reveals an ejection fraction of 32%, and the patient is referred to the cardiologist for management of systolic heart failure (Table 2).
Discussion of patient 1
Among BMT survivors, cardiovascular disease (CVD; eg, CHF, stroke, myocardial infarction) is 1 of the leading causes of nonrelapse mortality.21 BMT survivors have a fourfold increased risk of developing CVD when compared with the general population.22 Median age at first cardiovascular event is 53 years of age,7 lower than that observed in the general population (67 years of age).23 Pre-BMT anthracycline chemotherapy and/or mediastinal radiation are the best described risk factors for late-occurring CHF.4,5,7,21 In autologous BMT survivors, cumulative anthracycline dose ≥250 mg/m2 is associated with a 10-fold increased risk of CHF.5 BMT survivors have a twofold increased risk of developing hypertension, diabetes, or dyslipidemia as compared with age- and sex-matched controls.6,22 Exposure to total body irradiation (TBI) increases the risk for dyslipidemia and diabetes.6 The presence of hypertension among autologous BMT survivors with prior exposure to high-dose anthracyclines (≥250 mg/m2) increases the risk of CHF 35-fold when compared with those without anthracycline exposure or hypertension (Figure 2).5 Similarly, the risk of CHF is 27-fold increased for high-dose anthracycline recipients with diabetes (Figure 2).5 Thus, there is clear evidence that hypertension and diabetes serve as modifiers of anthracycline-related cardiac injury after BMT.5,22
Risk of congestive heart failure among 1244 autologous BMT recipients with and without hypertension and diabetes who received high-dose anthracyclines. The presence of hypertension among recipients of high-dose anthracyclines (≥250 mg/m2) resulted in a 35-fold increased risk of CHF; the risk was nearly 27-fold for high-dose anthracycline recipients with diabetes. DM, diabetes mellitus; HD, high-dose; HTN, hypertension. Adapted from Armenian et al.5
Risk of congestive heart failure among 1244 autologous BMT recipients with and without hypertension and diabetes who received high-dose anthracyclines. The presence of hypertension among recipients of high-dose anthracyclines (≥250 mg/m2) resulted in a 35-fold increased risk of CHF; the risk was nearly 27-fold for high-dose anthracycline recipients with diabetes. DM, diabetes mellitus; HD, high-dose; HTN, hypertension. Adapted from Armenian et al.5
In summary, the increased risk of CVD, coupled with the recognition that these complications develop earlier than would be expected in the general population, suggests an accelerated cardiovascular aging phenotype that may be initiated by pre-BMT therapeutic exposures and is worsened by post-BMT risk factors such as hypertension and diabetes.6 Recommendations for long-term monitoring18,20 range in intensity from a history and physical examination, with echocardiography reserved for clinical suspicion of cardiac compromise,18,24,25 to serial echocardiography through the life of a survivor, regardless of symptoms.26,27 Echocardiographic measures of cardiac function (eg, left ventricular ejection fraction, fractional shortening) are often derived from crude ventricular measurements and are late-occurring changes in the trajectory of disease.28 More sensitive markers of early cardiac dysfunction such as echocardiography-derived cardiac mechanics (eg, speckle tracking strain), tissue Doppler imaging, and 3-dimensional echocardiographic or cardiac magnetic resonance imaging have been incorporated into guidelines for evaluation of patients after cancer therapy.29,30 However, there have been no studies to demonstrate that early intervention based on change in subclinical markers of cardiac dysfunction, such as strain alone, can prevent the development of overt heart failure in cancer survivors. Given these limitations, a thorough history and physical examination (with attention to symptoms and signs of cardiac dysfunction, such as chest pain, shortness of breath, ankle swelling, decreased exercise tolerance, palpitations, and fainting/lightheadedness) should be part of the routine care of BMT survivors considered at high risk for CHF because of past exposure to cardiotoxic therapies.24 For individuals in whom there is concern for cardiac dysfunction, referral to a cardiologist is recommended. Importantly, BMT survivors should be educated regarding their long-term CVD risk, emphasizing the importance of early recognition of symptoms of cardiac dysfunction so that appropriate interventions (eg, angiotensin-converting enzyme inhibitors, β-blockers) can be initiated per established guidelines.24,31
Health care providers should regularly screen for and manage post-BMT cardiovascular risk factors such as hypertension and diabetes.18,24 In nononcology populations, aggressive management of hypertension (target systolic BP, <120 mm Hg)32 or diabetes (target hemoglobin A1C ≤6.5%)33 has been associated with decreased risk of CVD, a strategy worth considering in high-risk BMT survivors. There are emerging data among BMT survivors suggesting that adherence to a healthier lifestyle may attenuate risk of CVD; a diet rich in fruits and vegetables has been associated with reduced risk of diabetes and dyslipidemia, and increased physical activity has been associated with a decreased risk of hypertension.22 As such, adoption of a healthy lifestyle should be strongly encouraged for BMT survivors.
Clinical trials are needed to examine the timing and efficacy of interventions to reduce the risk of clinically overt cardiac disease in BMT survivors because the pathophysiology of these conditions often differ from that seen in nononcology populations. Such studies would help identify subsets of BMT patients who may derive the greatest benefit from more aggressive management of cardiovascular risk factors (eg, systolic BP <120 mm Hg, hemoglobin A1C ≤6.5%, low-density lipoprotein [LDL] <100 mg/dL, total cholesterol <200 mg/dL). Such efforts require multidisciplinary collaboration between PCPs, subspecialists (eg, cardiologists, endocrinologists), and BMT clinicians, allowing for personalized monitoring and intervention in survivors at highest risk for cardiac complications.
Monitoring for additional potential late complications
Therapeutic exposures place patient 1 at risk for pulmonary toxicity (bleomycin, carmustine, chest radiation), renal toxicity (ifosfamide, carboplatin), peripheral neuropathy (vinblastine, carboplatin), gonadal dysfunction (dacarbazine, ifosfamide, carmustine, melphalan, carboplatin), treatment-related myeloid neoplasm (t-MN; ifosfamide, melphalan, dacarbazine, carmustine, carboplatin, doxorubicin, etoposide), and subsequent solid neoplasms (chest radiation).
Pulmonary toxicity can develop with use of bleomycin, especially when chest radiation therapy and/or TBI are also part of the treatment. This toxicity develops months to years after completing therapy and usually manifests as cough and shortness of breath. High concentrations of oxygen exacerbates toxicity. One study found bleomycin lung damage in 18% of patients who received Adriamycin, bleomycin, vincristine, and dacarbazine for HL.34 For patients who have received potentially pulmonary toxic exposures (bleomycin, chest radiation, busulfan, nitrosoureas, TBI), we recommend pulmonary function tests at 1 year after BMT and then periodically as clinically indicated. Given the high risk for pulmonary infections in patients with a history of pulmonary toxic therapy, the pneumococcal vaccine series and yearly influenza vaccine are also recommended.
Renal toxicity can develop after BMT and may be associated with pretransplant exposures (ifosfamide, platinum-based chemotherapy, methotrexate, abdominal, and flank radiation), as well as TBI-containing BMT conditioning regimens, and exposures related to treatment of acute complications (eg, use of nephrotoxic antibiotics and antifungal agents) as well as ongoing treatment of GVHD (calcineurin inhibitors).35,36 Screening includes a renal function panel (serum creatinine, electrolytes) at 1 year following BMT, then as clinically indicated, and annual urinalysis for proteinuria, and annual BP monitoring.
Peripheral neuropathy associated with exposure to plant alkaloids (vincristine, vinblastine, taxanes) and heavy metals (carboplatin and cisplatin) is generally evident during or shortly after completion of therapy. Screening for at-risk patients should include a targeted history and physical examination with attention to the peripheral nervous system.37
Gonadal dysfunction and subsequent neoplasms (t-MN, solid tumors), and related monitoring are presented in detail under discussions for patients 3 and 4, respectively.
Patient 2
The patient is a 40-year-old female with a history of acute myeloid leukemia, French-American-British M-2 with t(8;21) diagnosed at the age of 25. She was treated with 3+7 induction chemotherapy (daunorubicin 270 mg/m2 and cytarabine 1.4 g/m2) followed by consolidation with idarubicin (36 mg/m2), fludarabine (72 mg/m2), and cytarabine (5.3g/m2). She subsequently underwent an allogeneic BMT from an HLA-identical unrelated donor, conditioned with cyclophosphamide (3.2 g/m2) and TBI (1320 cGy in 11 fractions). She has a history of chronic GVHD (cGVHD) of the skin, which required treatment for 18 months after transplantation with a combination of corticosteroids and sirolimus. She presented to the clinic with insidious onset of fatigue, weight gain, constipation, and cold intolerance. Physical examination was positive for a weight gain of 7 pounds over the past year, but unremarkable otherwise. Her blood work revealed a thyroid stimulating hormone (TSH) level of 7.176 mIU/L (normal reference range, 0.470-4.680) and free thyroxine (FT4) level of 0.51 ng/dL (0.66-2.01). The patient was diagnosed with hypothyroidism and thyroid hormone replacement therapy was initiated. In addition, the patient had laboratory evidence of dyslipidemia (total cholesterol, 285 mg/dL [50-199]; LDL, 192 mg/dL [30-129]; high-density lipoprotein, 41 mg/dL [40-59]; triglyceride, 275 mg/dL [10-149]), and was managed with cholesterol-lowering medications and diet (Table 3).
Discussion of patient 2
Thyroid abnormalities are well-described after BMT38-43 and include compensated hypothyroidism (elevated TSH accompanied with normal FT4 levels) and overt hypothyroidism (elevated TSH and low FT4). Approximately 30% of patients develop overt hypothyroidism over a 28-year follow-up period.41 Hypothyroidism is related to radiation to the thyroid gland (neck/mediastinal radiation or TBI); younger age at exposure increases the risk.42,43 Symptoms include fatigue, weight gain, constipation, dry skin, and menstrual irregularities. Screening for thyroid dysfunction relies on a good history and physical examination and annual thyroid function tests (TSH, FT4). Survivors with abnormalities on screening evaluation should be referred to an endocrinologist for hormone replacement therapy, which may be dependent upon whether the hypothyroidism is compensated or overt44 because some cases of compensated hypothyroidism resolve spontaneously over time.45 The risk of dyslipidemia is increased in patients with hypothyroidism, necessitating appropriate management of the thyroid condition to treat dyslipidemia.
Monitoring for additional potential late complications
Additional therapeutic exposures place patient 2 at risk for anthracycline-related cardiomyopathy; cardiovascular risk factors (TBI, corticosteroids, calcineurin inhibitors); pulmonary toxicity (TBI); renal toxicity (TBI, calcineurin inhibitors); osteonecrosis (TBI, corticosteroids, calcineurin inhibitors); t-MN (cyclophosphamide, daunorubicin, idarubicin); and radiation-related solid tumors (TBI). She is also at risk for gonadal dysfunction (cyclophosphamide, TBI), osteoporosis (corticosteroids, calcineurin inhibitors), neurocognitive dysfunction (TBI, high-dose cytarabine), and cataracts (corticosteroids, TBI).
Gonadal dysfunction.
It has been shown that almost all females age 13 years or older at receipt of fractionated TBI develop ovarian failure, probably as a result of decreased reserve of primordial follicles.46,47 Exposure to cyclophosphamide is also associated with gonadal failure.48 Screening for gonadal failure includes a detailed history (primary or secondary amenorrhea, menstrual irregularity, and pregnancies or problems with fertility), physical examination, and serum gonadotropin (luteinizing hormone [LH], follicle stimulating hormone [FSH]) and estradiol levels. High LH and FSH accompanied by low estradiol levels suggest primary ovarian failure. Irreversibility of ovarian function after BMT in most patients highlights the need for timely hormonal replacement therapy to prevent osteoporosis and other complications resulting from lack of gonadal hormones.
Osteoporosis.
BMT recipients are at risk for osteoporosis.9 The decreased bone mineral density seen in BMT survivors is typically resulting from treatment of GVHD with corticosteroids or calcineurin inhibitors49 ; physical inactivity and low dietary intake of calcium50 ; and/or uncorrected hypogonadism.51-53 Bone loss after BMT nadirs at 6 to 24 months.54,55 The incidence of osteoporosis approaches 20% at 2 years.56,57 Bone loss increases the risk of fractures; nontraumatic fractures have been observed in 10% of patients within 3 years after BMT.56 Transplant survivorship guidelines recommend assessment of bone mineral density with dual-energy x-ray absorptiometry (DXA) scan within 1 year after BMT, especially in those receiving allogeneic BMT and/or patients treated with prolonged corticosteroids and calcineurin inhibitors.18
Neurocognitive dysfunction.
TBI is associated with neurocognitive impairment, especially for those <50 years of age at exposure.58 The risk is also increased among those who received cranial radiation before BMT. Specific domains affected include executive functioning, attention, memory, and processing speed. Although neurocognitive function improves from 1 to 5 years after BMT, deficits remain for >40% of survivors.59 We recommend annual screening for cognitive/vocational difficulties and formal neuropsychological evaluation if difficulties are identified.
Cataracts.
Radiation (TBI or cranial radiation before BMT) and exposure to corticosteroids are associated with premature cataracts.60,61 The 10-year incidence of cataracts is higher among patients treated with single-dose TBI (60%), slightly lower among those who receive <6 fractions (43%), and significantly lower among those treated with >6 fractions (7%).62 Factors associated with increased risk of cataracts are older age, higher radiation dose rate (>0.04 Gy/min), allogeneic BMT, prolonged corticosteroids, and use of single-dose TBI.60-62 Annual ophthalmologic examination in patients at risk for cataracts is recommended.
Patient 3
The patient is a 25-year-old male diagnosed with stage IV Burkitt lymphoma at age 21. He was treated with French LMB-style therapy and received the following cumulative chemotherapy doses: cyclophosphamide (8.5 g/m2), methotrexate (32 g/m2), etoposide (2.1 g/m2), cytarabine (17.5 g/m2), doxorubicin (240 mg/m2), prednisone (780 mg/m2), vincristine (8 mg/m2), and triple intrathecal therapy. He was in remission for 11 months, but developed a recurrence, for which he received ifosfamide, 18 g/m2; carboplatin, 1.6 g/m2; and etoposide, 1 g/m2, followed by allogeneic BMT from an HLA-identical sibling donor (conditioning: cyclophosphamide [3.2 g/m2] and TBI [1320 cGy in 11 fractions]). He developed cGVHD of the skin, for which he has been receiving treatment with a combination of corticosteroids and sirolimus for the past 24 months. He presents to the clinic with complaints of pain in the left hip. Radiograph of the hip shows partial collapse of the left femoral head with sclerosis (Table 4).
Discussion of patient 3
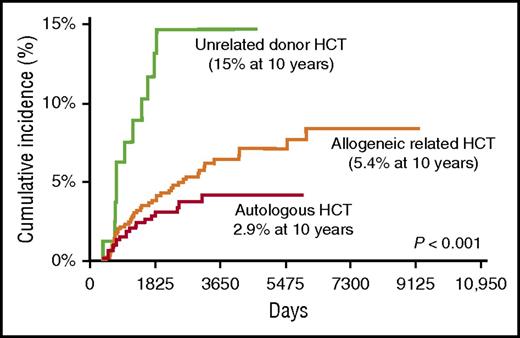
The complaint of left hip pain and the associated radiologic findings are consistent with osteonecrosis of the left femoral capital epiphysis, a complication associated with significant morbidity often requiring surgery. The risk of osteonecrosis is highest among unrelated donor recipients, approaching 15% at 10 years after BMT, and lowest among autologous BMT survivors, in whom the risk is less than 3% (Figure 3).8 The femoral head is most commonly affected; others include the knee and shoulder joints. Risk factors for osteonecrosis include male sex, GVHD therapy with corticosteroids or calcineurin inhibitors, and use of TBI for conditioning.8 The association with corticosteroids increases with cumulative dose.63 Clinical anticipation in at-risk survivors with a careful history and physical examination is the cornerstone of screening. Prompt radiologic evaluation with appropriate interventions can reduce the morbidity related to pain and lack of mobility.
Late osteonecrosis by BMT donor type in 1346 BMT recipients. The cumulative incidence of osteonecrosis was 2.9% after autologous BMT, 5.4% after allogeneic matched related BMT, and 15% after unrelated donor BMT. Adapted from Campbell et al.8
Late osteonecrosis by BMT donor type in 1346 BMT recipients. The cumulative incidence of osteonecrosis was 2.9% after autologous BMT, 5.4% after allogeneic matched related BMT, and 15% after unrelated donor BMT. Adapted from Campbell et al.8
Monitoring for additional potential late complications
Additional therapeutic exposures place patient 3 at risk for anthracycline-related cardiomyopathy, pulmonary toxicity (TBI), cardiovascular risk factors (TBI, calcineurin inhibitors, corticosteroids), renal toxicity (ifosfamide, carboplatin, methotrexate, TBI, calcineurin inhibitors), and peripheral neuropathy (vincristine, carboplatin); hypothyroidism (TBI), neurocognitive dysfunction (TBI, high-dose methotrexate and cytarabine), osteoporosis and related fractures (corticosteroids, calcineurin inhibitors, high-dose methotrexate), osteonecrosis (TBI, corticosteroids, calcineurin inhibitors), cataracts (TBI, corticosteroids), and t-MN (alkylators and topoisomerase II inhibitors) and radiation-related solid tumors. He is also at risk for gonadal dysfunction (cyclophosphamide, ifosfamide, carboplatin, TBI). Additionally, given the active cGVHD requiring immunosuppressive therapy, this patient is at risk for functional asplenia and associated life-threatening infections with encapsulated organisms and should receive vaccines specified in Table 4 and anticipatory health education regarding infection-related risks.64-66
Gonadal failure.
Exposure to cyclophosphamide (11.7 g/m2), ifosfamide (1 g/m2), and TBI (1320 cGy) places this patient at risk for gonadal failure. The risk of gonadal failure increases with cumulative doses of gonadotoxic therapies. Screening for gonadal failure includes a detailed history (assessing for decreased libido and fertility problems), serum gonadotropin and testosterone levels, and semen analysis (if the patient wishes to know his fertility status). High LH and FSH accompanied with low testosterone suggest primary gonadal failure. In men with low testosterone, referral to an endocrinologist for testosterone replacement therapy is indicated. In men with infertility or subfertility, referral to a reproductive endocrinologist may be indicated.
Patient 4
The patient is a 33-year-old female diagnosed with nodular sclerosing HL, stage IVB at age 18, presenting with bilateral supraclavicular and mediastinal disease, along with liver and spleen involvement. She was treated with cyclophosphamide (3.6 g/m2), vincristine (8.4 mg/m2), prednisone (3.4 g/m2), procarbazine (4.2 g/m2), doxorubicin (210 mg/m2), bleomycin (60 units/m2), and vinblastine (36 mg/m2). She had residual disease at the end of planned therapy. She underwent stem cell mobilization with etoposide (1.0 g/m2), cyclophosphamide (1.5 g/m2), and granulocyte colony-stimulating factor, followed by tandem peripheral blood stem cell transplant (conditioning: melphalan [150 mg/m2] and cyclophosphamide [3.3 g/m2]; etoposide [1.8 g/m2], and TBI [1200 cGy in 10 fractions]). She presented for a routine follow-up visit with a palpable 2-cm mass in the upper outer quadrant of the right breast. Mammography revealed focal asymmetry in the right upper outer region. High-resolution real-time ultrasound scan revealed an irregular hypervascular solid mass seen in the right breast at 10 o'clock position, corresponding to the palpable area. Biopsy revealed invasive ductal carcinoma. Tumor cells were positive for estrogen and progesterone receptors and were human epidermal growth factor receptor 2–positive (Table 5).
Discussion of patient 4
Exposure to chest radiation in pubertal females increases the risk of subsequent breast cancer.67 There is a linear dose-response relation between increasing radiation dose and risk of subsequent breast cancer.68 Whole-lung radiation also increases risk, demonstrating the importance of radiation volume in addition to dose.69 The association between TBI and subsequent breast cancer was examined in the setting of allogeneic BMT,70 placing allogeneic BMT survivors at a 2.2-fold increased risk of developing breast cancer when compared with age- and sex-matched general population. The median latency from BMT to breast cancer was 12.5 years. The incidence was higher among those exposed to TBI (17% vs 3%) and among those exposed to TBI at age <18 years.
SNs are classified into 3 distinct groups: (1) therapy-related myeloid neoplasms (t-MN); (2) lymphoma (including lymphoproliferative disorders); and (3) solid tumors.
t-MN.
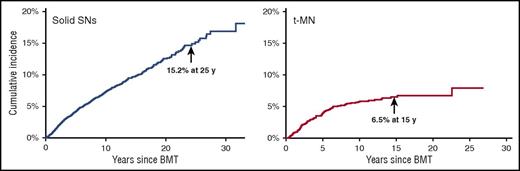
t-MN is a major cause of nonrelapse mortality after autologous BMT for HL and NHL.2,12 t-MN usually develops 4 to 7 years after alkylating agent exposure (Figure 4). Patients frequently present with cytopenias and multilineage dysplasia, with abnormalities involving chromosomes 5 (−5/del[5q]) and 7 (−7/del[7q]). t-MN secondary to topoisomerase II inhibitors presents as overt leukemia, without a preceding myelodysplastic phase. The latency is brief (6 months to 5 years) and is associated with balanced translocations involving chromosome bands 11q23 or 21q22. The risk of t-MN increases with age at BMT,3 pretransplantation therapy with alkylating agents, topoisomerase II inhibitors, radiation, use of peripheral blood stem cells, stem cell mobilization with etoposide, difficult stem cell harvests, conditioning with TBI, and (lower) number of CD34+ cells infused.2,3 The common and nonspecific nature of cytopenias after autologous BMT has resulted in the development of criteria for diagnosing t-MN after BMT: (1) significant marrow dysplasia in at least 2 cell lines, (2) unexplained peripheral cytopenias, and (3) blasts defined by French-American-British classification.71 Presence of a clonal cytogenetic abnormality in addition to morphologic criteria of dysplasia may aid in making this diagnosis.
Subsequent neoplasms after BMT from a single transplant center. The cumulative incidence of solid subsequent neoplasms is 15.2% at 25 years after autologous or allogeneic BMT. The cumulative incidence of t-MN is 6.5% at 15 years after autologous BMT. Adapted from Bhatia and Bhatia.80
Subsequent neoplasms after BMT from a single transplant center. The cumulative incidence of solid subsequent neoplasms is 15.2% at 25 years after autologous or allogeneic BMT. The cumulative incidence of t-MN is 6.5% at 15 years after autologous BMT. Adapted from Bhatia and Bhatia.80
Solid SNs.
In addition to subsequent breast cancer, our patient is also at risk for other solid SNs. Compared with an age- and sex-matched general population, BMT survivors carry a twofold increased risk of solid SNs.1 The risk of solid SNs increases over time (Figure 4).72 Solid SNs are associated with exposure to pre-BMT radiation or TBI and includes melanoma, basal cell carcinoma (BCC), salivary gland tumors, and thyroid, breast, bone, and connective tissue cancers, those related to cGVHD (squamous cell carcinoma [SCC] of the skin), or those related to chronic viral infection in the setting of immunosuppression (human papillomavirus: oropharyngeal or cervix cancer; and hepatitis B or C: hepatocellular carcinoma). A large study found the 20-year cumulative incidence of BCC and SCC as 6.5% and 3.4%, respectively, after allogeneic BMT.73 TBI increases the risk of BCC, particularly among young patients.74 Acute GVHD increases the risk of SCC; cGVHD increases the risk of both BCC and SCC.1 BMT recipients are at a threefold increased risk of thyroid cancer when compared with an age- and sex-matched general population.75 Age younger than 10 years at radiation, female sex, and cGVHD are associated with increased risk. Thyroid cancer develops after a latency of approximately 8 to 10 years.
Screening BMT survivors for SNs is important; however, evidence regarding frequency/intensity/modality remains limited. General recommendations are for the most part based on guidelines for the general population and certain high-risk subpopulations.76 Pre-BMT exposures (chest or cranial radiation, alkylating agents, topoisomerase II inhibitors) or BMT-related exposures (TBI, cGVHD) lead to elevated risks of SNs and must be considered in the screening strategies. We recommend annual history and physical examination, including oral cavity, uterine cervix, external genitalia, and full skin examination, colonoscopy every 5 years (minimum) beginning 10 years after radiation or age 35 years, and for those with palpable thyroid nodules, an ultrasound-guided fine needle aspiration. Specifically in regard to breast cancer screening recommendations for patient 4, COG guidelines indicate that semi-annual clinical breast examination and annual mammography and breast magnetic resonance imaging should be considered for patients with a history of TBI, beginning at age 25 or 8 years after radiation, whichever occurs last.19
Monitoring for additional potential late complications
Additional key therapeutic exposures place patient 4 at risk for cardiomyopathy (anthracycline), cardiovascular risk factors (TBI, prednisone), pulmonary toxicity (bleomycin, TBI), renal toxicity (TBI), and peripheral neuropathy (vinblastine, vincristine); gonadal dysfunction (cyclophosphamide, melphalan, procarbazine, TBI), hypothyroidism (TBI), neurocognitive dysfunction (TBI), osteoporosis and related fractures (prednisone), and cataracts (TBI, prednisone); as well as osteonecrosis (TBI, prednisone).
Conclusions
Exposure to pre-BMT treatment, high-intensity BMT-related treatment and immunosuppression after BMT increase the burden of morbidity after BMT. The adoption of maintenance-type therapy (eg, immunotherapy for lymphoma, lenalidomide for multiple myeloma) increases the risk of new complications (eg, hypogammaglobulinemia after rituximab77,78 ) that may develop after BMT.
This high burden of morbidity necessitates that strategies be developed for early detection of these complications. We have shown that although the vast majority of BMT survivors remain engaged with the health care system, the burden of providing complex and nuanced care falls upon PCPs because only a small minority of patients returns to the transplanting institutions for long-term care.79 This is not an ideal scenario because the PCPs are not equipped to handle these situations, nor are they comfortable in handling them because of time constraints as well as the challenge of staying abreast with issues faced by BMT survivors.
The care of the growing population of transplant survivors can be handled in 1 of 2 possible ways. The first option is to have specialized clinics for the long-term management of BMT survivors, offering risk-based screening for early detection of long-term complications. Several transplant programs offer this service to the survivors. The clinics often staffed by advanced practice professionals, offering risk-based screening for long-term complications. Patients are triaged to appropriate specialists if they are identified to have a complication. The second option is for dedicated PCPs with special interest in cancer survivorship issues to take on the responsibility of managing such a patient. A third option would be a hybrid approach in which the specialized clinics at transplant centers partner with PCPs in the community to optimize the long-term care of the transplant survivors.
No matter how or where BMT survivors receive long-term care, there is a need for standardized recommendations. It is important to note however that, although we do have evidence supporting the relationship between therapeutic exposures and long-term complications, we do not have evidence to support the frequency or modality of the screening tests. Instead, we rely upon consensus arrived by experts in the field. This is because limitations of sample size and/or long of complications preclude the ability to conduct randomized clinical trials to determine what frequency/intensity/modality would be superior in ensuring early detection or reduction in mortality.
Development of consensus-based guidelines represents a first step in standardizing long-term follow-up of BMT survivors. The overall goal of standardized guidelines is early identification of treatment-related complications, allowing for early intervention with resultant reduction in morbidity and mortality and attendant reduction in health care costs. To achieve the benefits of early detection and prevention of life-threatening complications, BMT survivors should undergo regular comprehensive physical examinations that include screening for functional and psychosocial consequences of treatment and education regarding key aspects of health promotion. The next important step is to ensure that the existing guidelines are disseminated and accepted by all health care providers caring for BMT survivors as well as by payers that reimburse for the costs of the screening tests. Adherence to standardized screening recommendations, the yield of screening, and the cost-effectiveness of standardized follow-up of BMT survivors will provide future evidence for guideline refinement.
Authorship
Contribution: S.B. conceived and designed the study; and S.B., S.H.A., and W.L. acquired, analyzed, and interpreted the data; wrote the manuscript; and gave final approval for the manuscript.
Conflict-of-interest disclosure. The authors declare no competing conflicts of interest.
Correspondence: Smita Bhatia, Institute for Cancer Outcomes and Survivorship, University of Alabama at Birmingham, 1600 7th Ave S, Lowder 500, Birmingham, AL 35233; e-mail: sbhatia@peds.uab.edu.