Key Points
CML progenitor cells demonstrate enhanced sensitivity to Wnt stimulation, related to increased FZD4 receptor expression.
Wnt inhibition by a Porcupine acyl transferase inhibitor enhances CML stem/progenitor cell targeting in combination with tyrosine kinase inhibition.
Abstract
Tyrosine kinase inhibitor (TKI) treatment of chronic myeloid leukemia (CML) has limited efficacy against leukemia stem cells (LSC) responsible for disease propagation, and most CML patients require continued TKI treatment to maintain remission. LSC maintenance is related, at least in part, to signals from the bone marrow microenvironment (BMM). Our previous studies have shown that Wnt signaling from the BMM contributes to preservation of CML LSC following TKI treatment. Secretion of Wnt ligands requires their modification by the O-acyl transferase Porcupine (PORCN). Here we investigated the activity of a potent and selective PORCN inhibitor, WNT974, against CML stem and progenitor cells. WNT974 efficiently antagonized Wnt signaling in human CML CD34+ cells, and in combination with the TKI nilotinib (NIL) significantly enhanced inhibition of proliferation and colony-forming potential of CML stem and progenitor cells and reduced their growth in immunodeficient mice in vivo, in comparison with NIL alone. Treatment of transgenic CML mice in vivo with NIL in combination with WNT974 significantly reduced leukemic stem and progenitor cell numbers, reduced regeneration of leukemic long-term hematopoietic stem cells in secondary transplant recipients, and enhanced survival of mice after discontinuation of treatment, in comparison with NIL alone. CML progenitors demonstrated enhanced sensitivity to Wnt stimulation, associated with increased expression of the FZD4 receptor. FZD4 knockdown inhibited CML progenitor growth. These results support further investigation of PORCN targeting to inhibit Wnt secretion and signaling and enhance targeting of CML stem cells while sparing their normal counterparts.
Introduction
Chronic myeloid leukemia (CML) results from transformation of a hematopoietic stem cell (HSC) by the BCR-ABL oncogene.1-3 Although BCR-ABL tyrosine kinase inhibitors (TKIs) are highly effective in treatment of chronic phase (CP) CML patients, leukemia stem cells (LSCs) are relatively resistant to TKI treatment, and the disease usually relapses upon treatment discontinuation.4 Because BCR-ABL kinase activity in CML LSCs is effectively inhibited following TKI treatment, alternative mechanisms may contribute to their maintenence.5-8 Normal HSCs are regulated by cells of the bone marrow (BM) microenvironment (BMM).9 There is increasing evidence that CML LSC may also be regulated by the BMM and that microenvironmental interactions may protect LSC from TKI treatment.10 There is considerable interest in developing strategies to target BMM-generated signals supporting LSC.11-14
Wnts are secreted glycoproteins that activate signaling cascades that regulate embryonic development, cell differentiation, and proliferation.15 The Wnt pathway includes 19 different Wnt ligands, 10 Frizzled (FZD) receptors, and multiple signaling intermediates. The best studied Wnt signaling cascade is the “canonical” β-catenin-dependent pathway. Wnt ligand binding to lipoprotein receptor-related protein (LRP)5/6 and FZD receptors triggers disruption of the β-catenin destruction complex, β-catenin translocation to the nucleus, interaction with the LEF/TCF transcription factors, and expression of Wnt target genes.16 The impact of Wnt signaling in hematopoiesis is influenced by developmental stage, signal strength, and microenvironmental factors.17-26 Constitutive deletion of CTNNB1 compromises long-term maintenance of HSC, whereas conditional inactivation of CTNNB1 in adult mice does not alter HSC repopulation and self-renewal, suggesting a critical role of β-catenin in embryonic but not adult HSC.27,28 The level of Wnt signaling may affect the balance of HSC self-renewal versus differentiation, with low levels contributing to HSC maintenance and increased hematopoietic reconstitution, and high levels hindering HSC self-renewal and differentiation. Overexpression of Wnt inhibitors such as Dickkopf1 (Dkk1) or Wnt inhibitory factor 1 (Wif1) reduces HSC quiescence and self-renewal.
Studies in CML have shown that β-catenin signaling is constitutively activated in blast crisis.29 Enhanced β-catenin activity may result from GSK3β missplicing, BCR-ABL-mediated inactivation of GSK3β function and phosphorylation of β-catenin, or abnormal Mnk signaling. 16,30-32 Transplantation of BCR-ABL-transduced HSC from β-catenin knockout mice led to delayed onset of leukemia and loss of LSC self-renewal.17 These studies, although showing an important role for β-catenin, do not address the role of microenvironmental Wnt signaling in CML LSC maintenance. We have previously shown that N-cadherin-mediated adhesion of CML LSC to mesenchymal stromal cells (MSC) increases β-catenin levels, enhances nuclear translocation of β-catenin, and increases transcription of Wnt target genes.33 These studies suggested that microenvironmental Wnt signaling supports maintenance of CML LSC.
Palmitoylation of Wnts by the endoplasmic reticulum membrane-bound O-acyl transferase Porcupine (PORCN) is required for their secretion and activity.34,35 WNT974 is a selective PORCN inhibitor that potently and selectively inhibits Wnt signaling and targets Wnt-dependent solid tumors.36,37 Here we investigated the ability of WNT974 to inhibit Wnt signaling in CML stem and progenitor cells, inhibit their growth, and enhance their targeting by the TKI nilotinib (NIL).
Materials and methods
Samples
Samples were obtained from CP CML patients seen at City of Hope (COH) or the University of Alabama at Birmingham (UAB). Cord blood samples (normal) were provided by StemCyte (Arcadia, CA). All subjects signed informed consent. Sample acquisition was approved by institutional review boards at COH and UAB, in accordance with assurances filed with the US Department of Health and Human Services, and met all requirements of the Declaration of Helsinki.
Cell culture
We irradiated human reverse transcriptase-immortalized primary human BM MSCs38 subcultured in tissue culture plates at 20 Gy. CD34+ cells were cultured in the presence or absence of irradiated MSCs in StemSpan serum-free expansion medium (Stem Cell Technologies, Vancouver, BC, Canada), supplemented with growth factors at concentrations found in stroma-conditioned medium from long-term BM cultures39 at 37°C in 5% CO.2
Wnt-β-catenin reporter activity
A β-catenin–activated reporter (BAR) reporter containing 12-TCF-response DNA-binding elements that drive transcription of firefly luciferase (pBARLS) was used.40 A control reporter (pfuBARLS) with a 2-base substitution in each TCF-binding site acted as a negative control. A pSL9/Ren plasmid with renilla luciferase driven by the EF1a promoter was used as an internal control. Luciferase was quantified using the Dual-Luciferase Reporter Assay System (catalogue no. E1910; Promega, Madison, WI), and data were expressed as ratio of firefly to renilla units and then as ratio of pBARLS to pfuBARLS.
Palmitoylation assay
HEK 293T cells overexpressing WNT1 were treated with WNT974 for 24 h, metabolically labeled with azide-containing palmitic acid for 6 h, and modified palmitoylated proteins detected by labeling with alkyne-containing allophycocyanin (APC) dye using flow cytometry.
Immunofluorescence microscopy
CML CD34+ cells were fixed, permeabilized, and incubated with mouse anti–β-catenin antibody (14/b-catenin; BD Biosciences), followed by fluorescein isothiocyanate–conjugated anti-mouse secondary antibody and 4′,6-diamidino-2-phenylindole (DAPI). Slides were examined using a Zeiss upright LSM 510 confocal microscope.
Real-time quantitative polymerase chain reaction (qPCR) analysis
Total RNA was extracted using RNAeasy Mini (Qiagen, Valencia, CA), and cDNA was synthesized using the Superscript III First-Strand Kit (Invitrogen, Grand Island, NY). qPCR for BCR-ABL and BCR was performed, as has been previously described.6 TaqMan gene expression assays were used for qPCR for MYC (Hs00153408_m1), CCND1 (Hs00765553_m1), PPARD (Hs04187066_g1), AXIN2 (Hs00610344_m1), FZD1 (Hs00268943_s1), FZD4 (Hs00201853_m1), FZD5 (Hs00258278_s1), and ACTB (Hs01060665_g1). β-2M assays were described previously.41
Western blotting
Western blotting was performed as previously described.33 Antibodies used included anti–β-catenin (14/b-catenin; BD Biosciences), anti–p-LRP6 (S1490; Cell Signaling, Danvers, MA), anti–total LRP6 (Cell Signaling), anti-actin (clone: AC15; Sigma Aldrich), anti–glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (clone: GAPDH-71.1; Sigma Aldrich), anti-ROR2 (clone: H-1; Santa Cruz Biotechnology, Santa Cruz, CA), anti-frizzled-1 (clone: V-22; Santa Cruz), anti-frizzled-4 (Abcam, Cambridge, MA), anti-frizzled-5 (Abcam), and Horseradish peroxidase–conjugated secondary antibodies (Jackson ImmunoResearch Laboratories, West Grove, PA). Antibody detection was performed using the SuperFemto kit (Pierce Biotechnology, Rockford, IL).
Analysis of apoptosis, proliferation, and colony-forming cell assays
Apoptosis was assessed by labeling cells with Annexin V-APC (BD Biosciences PharMingen, San Diego, CA) and DAPI. Proliferation was assessed by labeling cells with 5- (and 6-) carboxy-fluorescein diacetate succinimidyl ester (CFSE; Molecular Probes, Eugene, OR). Cells were cultured for 6 days and CFSE analyzed by flow cytometry. ModFit software (Verity, Topsham, ME) was used to fit data, determine percentage of cells in each generation, and generate a proliferation index. The position of the parent generation was based on cell aliquots treated with 4% paraformaldehyde immediately after sorting. Colony-forming cell (CFC) assays were performed by plating cells in methylcellulose progenitor culture (Stem Cell Technologies, Vancouver, BC, Canada). After 14 days, colony-forming unit-granulocyte, erythroid, macrophage, and megakaryocyte; colony-forming unit-granulocyte and macrophage; and burst-forming unit-erythroid were counted.
Engraftment of human cells in immunodeficient mice
For ex vivo treatment, CML CD34+ cells (2 × 106 cells per mouse) treated with WNT974, NIL, or the combination of the two, were transplanted via tail-vein injection into sublethally irradiated (3 Gy) nonobese diabetic, severe combined immunodeficiency IL2RƔnull-3 (NSG; Jackson Laboratory, Bar Harbor, ME) mice, and human cell engraftment was assessed after 16 weeks by flow cytometry after labeling with antibodies to human CD45, CD34, CD33, CD14, CD15, CD3, and CD19. BCR-ABL messenger RNA (mRNA) levels in BM cells were evaluated by qPCR. For in vivo treatment, CML CD34+ cells were transplanted into NSG mice, engraftment checked after 4 weeks, and mice treated with vehicle, WNT974 (5mg/kg twice daily), NIL (50 mg/kg once daily), or the combination of the two by oral gavage for 3 weeks and human cell engraftment examined. Human CD45+ cells were selected using immunomagnetic columns and analyzed for CFC frequency. Fluorescence in situ hybridization (FISH) analysis was performed using the BCR-ABL dual-fusion probe.
Plasma concentration of drugs
Normal healthy wild-type Friend virus B/NIH (FVB/N) mice (n = 5 per dosing group) were treated by oral gavage (WNT974 twice a day; NIL once a day). The plasma concentrations and exposures of WNT974 and NIL in mice were determined on day 14. Blood samples were collected at 2, 4, 10, and 24 h after last dose and analyzed by liquid chromatography/mass spectrometry (MS)/MS.
Treatment of transgenic BCR-ABL mice
Transgenic BCR-ABL mice were maintained on tetracycline.42-44 BM cells (CD45.1) obtained 3 weeks after induction of BCR-ABL expression by tetracycline withdrawal were transplanted (106 cells/mouse) into CD45.2 FVB/N recipient mice irradiated at 750 cGy. After 3 weeks, mice were treated with NIL, WNT974, or the combination of the two by oral gavage for 2.5 weeks. Donor CD45.1 mature myeloid, progenitor, and stem cell populations in peripheral blood (PB), BM, and spleen were measured by flow cytometry.42 Antibodies included anti-mouse CD45.1, Gr1Mac-1, Ter119, CD3, NK1.1, IgM, CD4, CD8a, B220, Gr-1, CD11b, CD19, interleukin-7Rα, CD34, FcγR, Sca-1, c-Kit, CD150, CD48, and antistreptavidin (e-Biosciences). Populations analyzed were granulocyte-macrophage progenitors (GMP; Lin−Sca-1−c-Kit+CD34+FcγR+), common myeloid progenitors (CMP; Lin−Sca-1−c-Kit+CD34+FcγR−), LSK (Lin−Sca-1+c-Kit+), long-term hematopoietic stem cells (LTHSC; Lin−Sca-1+c-Kit+CD150+CD48−), short-term hematopoietic stem cells (STHSC; Lin−Sca-1+c-Kit+CD150−CD48−), multipotent progenitors (MPP1; Lin−Sca-1+c-Kit+CD150+CD48+; MPP2, Lin−Sca-1+c-Kit+CD150−CD48+). For secondary transplantation, BM cells from treated mice were pooled and transplanted (2 × 106 cells/mouse) into irradiated FVB/N recipient mice, and mice were analyzed after 12 weeks. Another subset of mice was followed for survival after discontinuation of treatment. All experimental procedures were carried out in accordance with federal guidelines and protocols approved by COH and UAB institutional animal care and use committees.
Small interfering RNA (siRNA) transfection
CML CD34+ cells were transfected with Silencer Select siRNAs to FZD1 (clone: s15836; Ambion), FZD4 (clone: s15840; catalogue no.: 4390824; Ambion), FZD5 (clone: s15416; Ambion), and negative control nontargeting siRNAs (Silencer Select Negative Control No. 1; Ambion) using nucleofection (Amaxa Biosystems, Gaithersburg, MD). All experiments included 6-carboxyfluorescein–conjugated siRNA as a transfection control. Gene knockdown was confirmed 48 h posttransfection by western blot and qPCR. pBAR reporter stably expressing K562 cells were transfected with respective siRNAs and luciferase activity quantified using the Dual-Luciferase system.
Statistical analysis
Results are displayed as means plus/minus standard errors of the means (SEM). Significance values were calculated using Prism version 5.0 software (GraphPad Prism, La Jolla, CA) using unpaired, nonparametric t tests (Mann-Whitney test) or 2-way analysis of variance as appropriate. Survival was analyzed using Kaplan-Meier analysis.
Results
WNT974 inhibits Wnt signaling in human CML stem/progenitor cells
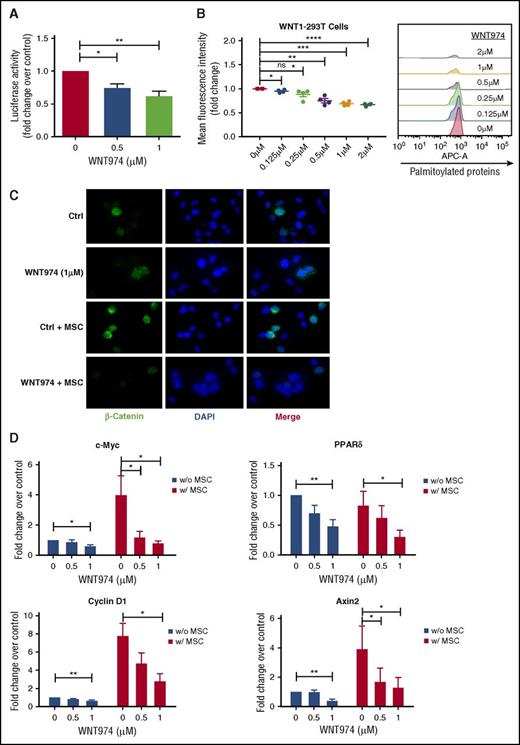
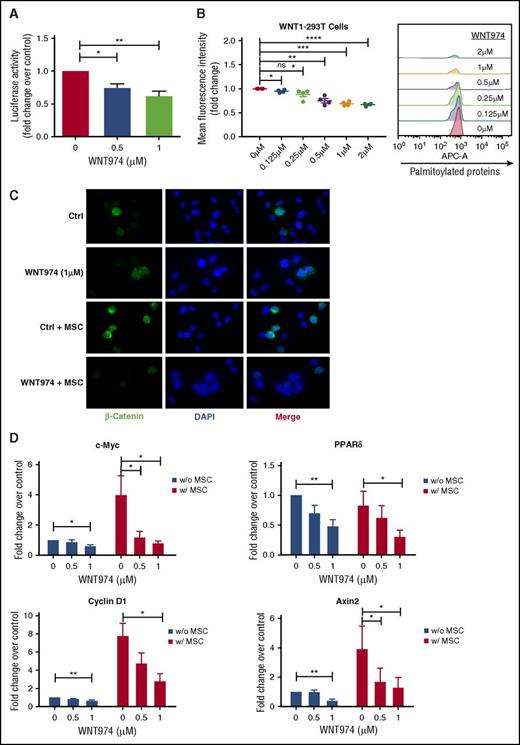
Although the IC50 of PORCN inhibition by WNT974 is 0.3 nM, drug sensitivity is cell context dependent, and in several cell types inhibition of Wnt signaling requires concentrations up to 1 µM.36,37,45 To evaluate inhibition of Wnt secretion, we treated Wnt1-overexpressing MSC with increasing concentrations of WNT974, and we tested conditioned medium on 293T cells expressing a pBARLS Wnt reporter. Reporter activity was significantly reduced with WNT974 treatment (0.5, 1.0 µm) (Figure 1A). We further show that WNT974 treatment resulted in reduced incorporation of palmitic acid molecules into proteins, confirming blockade of palmitoylation activity (Figure 1B). Immunofluorescence analysis indicated that MSC coculture resulted in increased expression of β-catenin in CML progenitors, as previously described33 (Figure 1B; supplemental Figure 1A-C, available on the Blood Web site). WNT974 (1 µM) reduced intensity and nuclear translocation of β-catenin, both with or without MSC (Figure 1B; supplemental Figure 1A-C). WNT974 treatment (0.5, 1.0 µm) significantly decreased expression of the Wnt target genes c-Myc, Cyclin D1, PPARδ, and Axin2 in CML progenitors (Figure 1D), and reduced expression of ROR2, a coreceptor required for noncanonical Wnt signaling, in K562 cells (supplemental Figure 1D). These results indicate that WNT974 inhibits both autocrine and paracrine Wnt signaling in CML cells.
WNT974 antagonizes the Wnt signaling pathway in human CML stem/progenitor cells. (A) Wnt secretion was evaluated in WNT1-MSC cultured in the presence of WNT974 for 24 h. Conditioned medium was harvested and added onto 293T-BAR reporter cells. WNT-β-catenin transcriptional activity was then evaluated after a further 24 h (n = 5). (B) HEK 293T cells overexpressing Wnt-1 were treated with WNT974, metabolically labeled with azide-containing palmitic acid and modified palmitoylated proteins detected by labeling with alkyne-containing APC dye using flow cytometry (n = 4). (C) CML CD34+ cells were cultured in the presence or absence of human MSC in the presence of WNT974 for 48 h, and immunofluorescence microscopy was performed. CML CD34+ cells labeled with antibodies to β-catenin (green) and DAPI (blue) are shown. Two samples were studied. All scale bars represent a size of 10 µM, and at least 200 cells were analyzed for each sample. (D) qPCR analysis for mRNA expression of Wnt target genes in CML CD34+ cells (n = 5) cultured as in panel A. Error bars represent mean ± SEM. Ctrl, control; ns, not significant; PPAR, peroxisome proliferator-activated receptors. *P < .05; **P < .01; ***P < .001; ****P < .0001.
WNT974 antagonizes the Wnt signaling pathway in human CML stem/progenitor cells. (A) Wnt secretion was evaluated in WNT1-MSC cultured in the presence of WNT974 for 24 h. Conditioned medium was harvested and added onto 293T-BAR reporter cells. WNT-β-catenin transcriptional activity was then evaluated after a further 24 h (n = 5). (B) HEK 293T cells overexpressing Wnt-1 were treated with WNT974, metabolically labeled with azide-containing palmitic acid and modified palmitoylated proteins detected by labeling with alkyne-containing APC dye using flow cytometry (n = 4). (C) CML CD34+ cells were cultured in the presence or absence of human MSC in the presence of WNT974 for 48 h, and immunofluorescence microscopy was performed. CML CD34+ cells labeled with antibodies to β-catenin (green) and DAPI (blue) are shown. Two samples were studied. All scale bars represent a size of 10 µM, and at least 200 cells were analyzed for each sample. (D) qPCR analysis for mRNA expression of Wnt target genes in CML CD34+ cells (n = 5) cultured as in panel A. Error bars represent mean ± SEM. Ctrl, control; ns, not significant; PPAR, peroxisome proliferator-activated receptors. *P < .05; **P < .01; ***P < .001; ****P < .0001.
WNT974 in combination with nilotinib inhibits growth of human CML progenitor cells
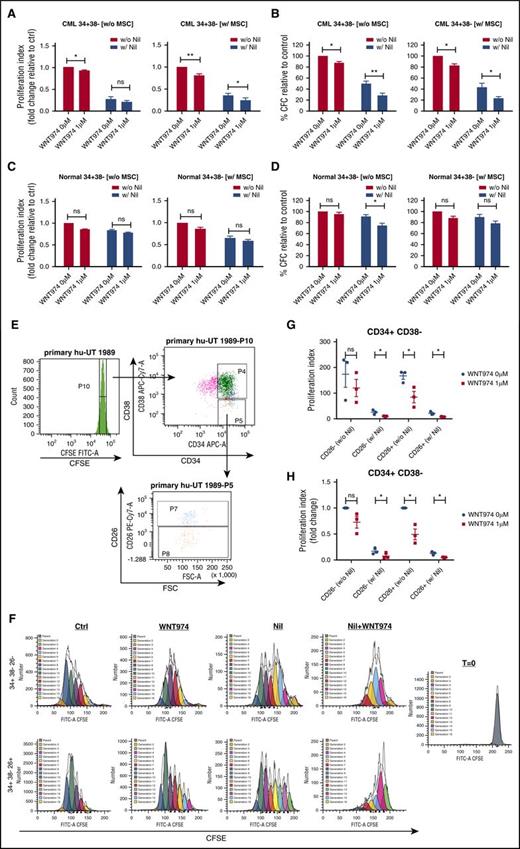
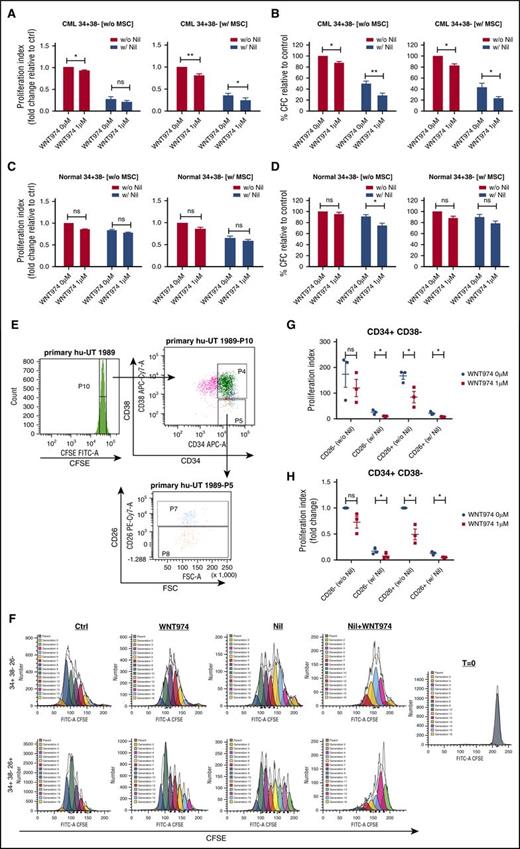
WNT974 did not enhance apoptosis in CML and normal CD34+ progenitors (supplemental Figure 2A-D) but resulted in significant dose-dependent reduction of CFC capacity of CML in comparison with normal CD34+ cells, both in the absence and presence of MSC (supplemental Figure 2E-H). Combined WNT974 and NIL treatment significantly reduced proliferation of CML CD34+CD38− and CD34+CD38+ cells in comparison with NIL alone in the presence of MSC (Figure 2A; supplemental Figure 3A). Proliferation of normal CD34+CD38− and CD34+CD38+ cells was unaffected (Figure 2C; supplemental Figure 3B). CD26 expression enriches for LSC within CML CD34+CD38− cells46 (Figure 2). WNT974 significantly inhibited proliferation of CD34+CD38−CD26+ cells, but not CD34+CD38−CD26− cells, indicating increased activity against this LSC-enriched population. The combination of WNT974 and NIL treatment significantly inhibited both subpopulations in comparison with NIL (Figure 2F-H). WNT974 significantly reduced CFC capacity of CML CD34+CD38− cells both in the absence and presence of MSC (Figure 2B). The colony-forming ability of CML 34+38+ cells was inhibited in the presence of MSC but not without MSC (supplemental Figure 3C). WNT947 combined with NIL inhibited the CFC capacity of CML CD34+CD38− and CD34+CD38+ cells to a significantly greater extent than NIL alone, and compared with their normal counterparts (Figure 2D; supplemental Figure 2D). These results suggest that inhibition of Wnt secretion in combination with NIL enhances inhibition of proliferation and clonogenic potential of CML stem/progenitor cells.
WNT974 in combination with nilotinib inhibits the proliferation and clonogenic potential of human CML primitive and committed progenitor cells. Normal and CML 34+ cells (n = 4-5) were labeled with CFSE. CFSE+ primitive cells (34+38−) and committed cells (34+38+) were sorted by flow cytometry and cultured in the presence or absence of MSC for 6 days with WNT974, Nil (1 µM), or both, or left untreated. A proliferation index was calculated on the basis of reduction in CFSE levels for CML 34+38− cells (A) and for normal 34+38− cells (B). Cells were also plated in methylcellulose progenitor assays, and colony-forming cell (CFC) frequencies were determined after 14 days for CML 34+38− cells (C) and for normal 34+38− cells (D). CML 34+ cells (n = 3) were labeled with CFSE, CD34, CD38, and CD26, and CD34+CD38−CD26+ and CD34+CD38−CD26− cells were selected by fluorescence-activated cell sorting (E) and cultured in the presence of MSC for 6 days with WNT974, NIL, or both, or left untreated. (F) Example CFSE plots of one CML sample are shown. Proliferation index was measured on the basis of reduction of CFSE levels and both actual values (G), and fold change in relation to untreated controls (H) are shown. Error bars represent mean ± SEM. FSC, forward scatter; Nil, nilotinib; w, with; w/o, without. *P < .05; **P < .01.
WNT974 in combination with nilotinib inhibits the proliferation and clonogenic potential of human CML primitive and committed progenitor cells. Normal and CML 34+ cells (n = 4-5) were labeled with CFSE. CFSE+ primitive cells (34+38−) and committed cells (34+38+) were sorted by flow cytometry and cultured in the presence or absence of MSC for 6 days with WNT974, Nil (1 µM), or both, or left untreated. A proliferation index was calculated on the basis of reduction in CFSE levels for CML 34+38− cells (A) and for normal 34+38− cells (B). Cells were also plated in methylcellulose progenitor assays, and colony-forming cell (CFC) frequencies were determined after 14 days for CML 34+38− cells (C) and for normal 34+38− cells (D). CML 34+ cells (n = 3) were labeled with CFSE, CD34, CD38, and CD26, and CD34+CD38−CD26+ and CD34+CD38−CD26− cells were selected by fluorescence-activated cell sorting (E) and cultured in the presence of MSC for 6 days with WNT974, NIL, or both, or left untreated. (F) Example CFSE plots of one CML sample are shown. Proliferation index was measured on the basis of reduction of CFSE levels and both actual values (G), and fold change in relation to untreated controls (H) are shown. Error bars represent mean ± SEM. FSC, forward scatter; Nil, nilotinib; w, with; w/o, without. *P < .05; **P < .01.
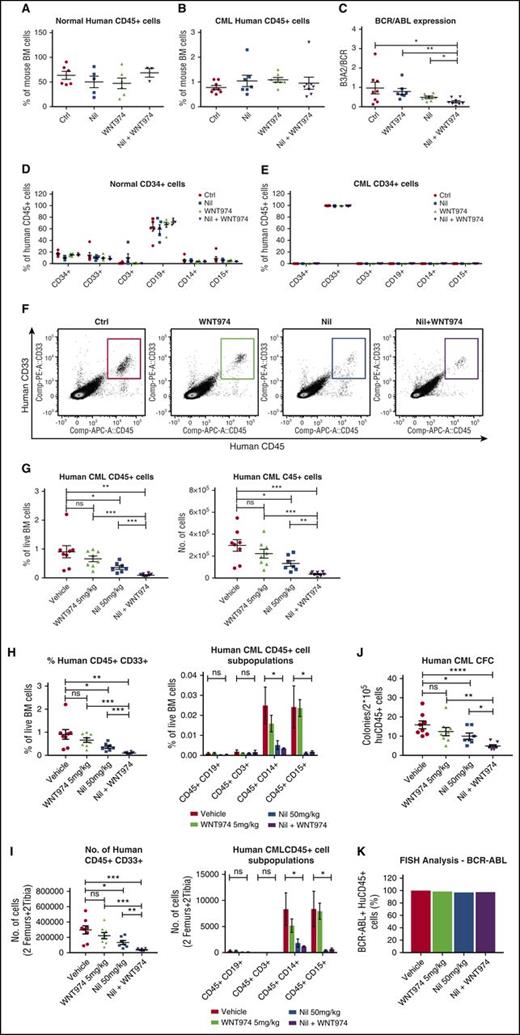
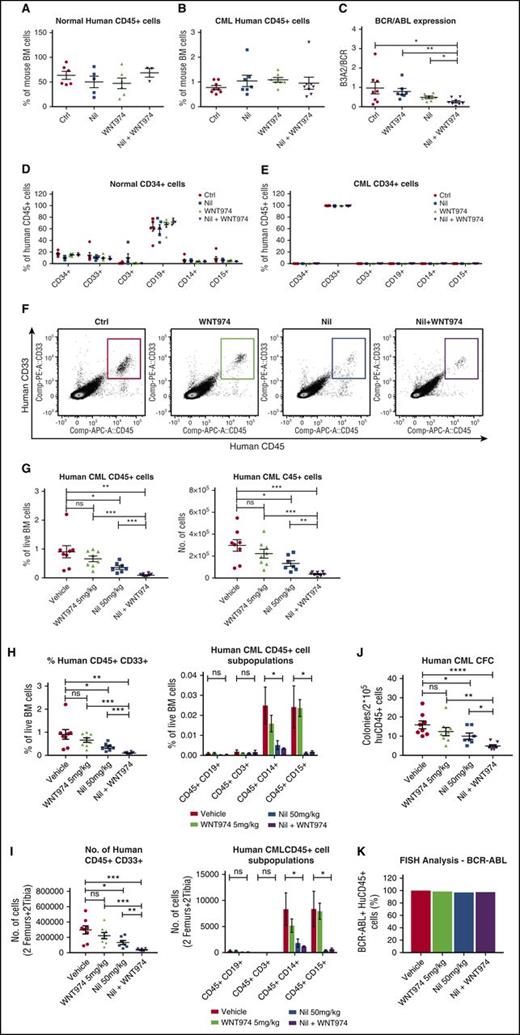
To test effects on in vivo repopulating potential, we cultured CML and normal CD34+ cells on MSC with WNT974, NIL, or the combination of the two for 4 days and transplanted them into NSG mice. Long-term engraftment of normal CD34+ cells was unaffected by drug treatment (Figure 3A,D). Treatment of CML CD34+ cells with NIL, WNT974, or the combination of the two did not affect total CD45+ cell engraftment (Figure 3B,E), but WNT974 plus NIL treatment resulted in significantly reduced BCR-ABL mRNA levels in engrafted human CD45+ cells (Figure 3C), suggesting reduction in BCR−ABL+ in relation to nonmalignant cells. To investigate the effect of WNT974 in vivo, we transplanted CML CD34+ cells (2 × 106 cells/mouse) into sublethally irradiated NSG mice. Human CML engraftment in blood was checked after 4 weeks, and mice were treated with vehicle, WNT974 (5 mg/kg twice daily), NIL (50 mg/kg daily), or both for 3 weeks (n = 7-8 mice per condition). The WNT974 and NIL combination significantly reduced the frequency and absolute number of human CD45+ cells (Figure 3F-G), myeloid progenitors (human CD33+), and mature myeloid cells (CD14+, CD15+) in the BM in comparison with vehicle and individual drug-treated mice (Figure 3H,I). The CFC-forming ability of human CD45+ cells from mice treated with WNT974 in combination with NIL was significantly reduced in comparison with vehicle and individually treated mice (Figure 3J). FISH analysis confirmed that engrafted cells were BCR−ABL+ (Figure 3K). These results indicate that the WNT974 and NIL combination inhibits BCR−ABL+ CML LSC with in vivo repopulating capacity in comparison with NIL alone.
WNT974 in combination with nilotinib inhibits the long-term engraftment of human CML stem cells in immunodeficient mice without affecting normal stem cells. Normal (1 × 105 cells per mouse) and CML 34+ cells (2 × 106 cells per mouse) were cultured in the presence of MSC and treated with nilotinib (Nil) (1 µM), WNT974 (1 µM), or both, or were untreated for 4 days; they were then transplanted into NSG mice (6-8 mice per group). Mice were killed after 16 weeks, and bone marrow (BM) content of femurs was obtained. Graph showing normal human CD45+ cell engraftment in BM (A); human CML CD45+ cell engraftment in BM (B); BCR-ABL mRNA levels in CML CD45+ cells engrafted in BM (C); lineage marker expression of normal CD45+ hematopoietic cells engrafted in BM (D); and lineage marker expression of CML CD45+ hematopoietic cells engrafted in BM (E). In a second experiment, CML CD34+ cells were transplanted into sublethally irradiated NSG mice, and 3 weeks posttransplantation, mice were treated with NIL (50 mg/kg), WNT974 (5 mg/kg), or the combination of these for 3 weeks. Representative results of fluorescence-activated cell sorter analysis of BM cells (F), frequency and absolute number of total human CD45+ cells (G), and aggregate results of the percentage (H) and absolute number (I) of human myeloid progenitors and lineage marker expression in the BM from treated mice are shown. (J) Human CD45+ cells were selected and plated in CFC assays. (K) FISH analysis was performed on interphase nuclei of 200 CD45+ cells from each treatment arm. Error bars represent mean ± SEM. *P < .05; **P < .01; ***P < .001; ****P < .0001.
WNT974 in combination with nilotinib inhibits the long-term engraftment of human CML stem cells in immunodeficient mice without affecting normal stem cells. Normal (1 × 105 cells per mouse) and CML 34+ cells (2 × 106 cells per mouse) were cultured in the presence of MSC and treated with nilotinib (Nil) (1 µM), WNT974 (1 µM), or both, or were untreated for 4 days; they were then transplanted into NSG mice (6-8 mice per group). Mice were killed after 16 weeks, and bone marrow (BM) content of femurs was obtained. Graph showing normal human CD45+ cell engraftment in BM (A); human CML CD45+ cell engraftment in BM (B); BCR-ABL mRNA levels in CML CD45+ cells engrafted in BM (C); lineage marker expression of normal CD45+ hematopoietic cells engrafted in BM (D); and lineage marker expression of CML CD45+ hematopoietic cells engrafted in BM (E). In a second experiment, CML CD34+ cells were transplanted into sublethally irradiated NSG mice, and 3 weeks posttransplantation, mice were treated with NIL (50 mg/kg), WNT974 (5 mg/kg), or the combination of these for 3 weeks. Representative results of fluorescence-activated cell sorter analysis of BM cells (F), frequency and absolute number of total human CD45+ cells (G), and aggregate results of the percentage (H) and absolute number (I) of human myeloid progenitors and lineage marker expression in the BM from treated mice are shown. (J) Human CD45+ cells were selected and plated in CFC assays. (K) FISH analysis was performed on interphase nuclei of 200 CD45+ cells from each treatment arm. Error bars represent mean ± SEM. *P < .05; **P < .01; ***P < .001; ****P < .0001.
WNT974 in combination with nilotinib significantly depletes leukemic stem and progenitor cells in BCR-ABL mice
The low levels of engraftment of CML CP cells in NSG mice limit the use of this model to evaluate CML LSC. An inducible BCR-ABL transgenic mouse model of CML provides a representative and well-established model of CP CML.42,44,47-50 The functional capacity of stem and progenitor populations in this model are well characterized, with only cells with LTHSC phenotype capable of long-term engraftment and leukemia generation.42 This model has been extensively used to study the effects of treatments on LSC.42,51-55
Plasma concentrations of drugs were measured in healthy FVB/N mice treated with NIL (50 mg/kg), WNT974 (5 mg/kg), or the combination of the two. Although NIL levels were similar in mice treated with NIL alone or in combination with WNT974, levels of WNT974 were increased in mice treated with WNT974 plus NIL in comparison with WNT974 alone (supplemental Figure 4A-B). Also, twice-daily administration of WNT974 was required to sustain drug levels.
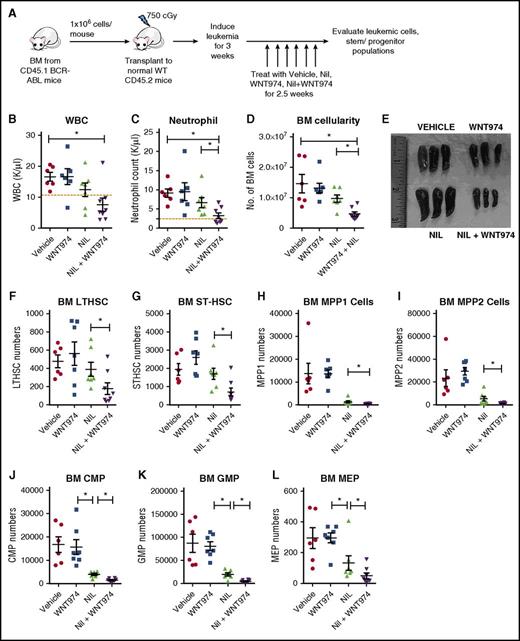
Transplantation of leukemic BM cells (CD45.1) into wild-type CD45.2 FVB/N mice results in the consistent development of a myeloproliferative disorder and allows generation of large cohorts with similar time of onset of leukemia, facilitating therapeutic studies. A cohort of CML mice were treated with WNT974 (5 mg/kg twice daily), NIL (50 mg/kg daily), or the combination of the two for 2.5 weeks (Figure 4A). WNT974 in combination with NIL significantly reduced total white blood cells (WBC), neutrophils, and myeloid cells in PB in comparison with NIL alone (Figure 4B-C; supplemental Figure 4F). The combination significantly reduced BM cellularity, and spleen size, cellularity, and weight (Figure 4D-E; supplemental Figure 4G-H), and enhanced reduction of LTHSC and in the BM and spleen in comparison with vehicle or NIL alone (Figure 4F; supplemental Figure 4I). STHSC, MPP1, MPP2, CMP, GMP, and megakaryocyte-erythroid progenitor (MEP) were significantly reduced in BM of combination-treated mice in comparison with vehicle or NIL-treated mice (Figure 4G-L). Combination therapy also significantly reduced the numbers of MPP2, CMP, GMP, and MEP in the spleen (supplemental Figure 4J-O). We observed significant inhibition of c-Myc, Cyclin D1, and Axin2 (supplemental Figure 4C-E) expression by real-time qPCR in BM c-Kit+ cells from mice treated with WNT974 and NIL plus WNT974 in comparison with vehicle-treated mice.
WNT974 in combination with nilotinib significantly reduces leukemic stem and progenitor cells in the BM and spleen of transgenic BCR-ABL mice. (A) BCR-ABL expression was induced in SCL-tTA-BCR-ABL mice by tetracycline withdrawal. BM cells were obtained 3 weeks after induction and transplanted into wild-type FVB/N recipient mice irradiated at 750 cGy (106 cells/mouse; 6-8 mice/group). Three weeks posttransplantation, treatment with vehicle, NIL (50 mg/kg), WNT974 (5 mg/kg), or a combination was initiated. PB WBC counts (B), PB neutrophil counts (C), BM cellularity (D), spleen size (E), absolute number of LTHSC (F), STHSC (G), MPP1 (H), MPP2 (I), CMP (J), GMP (K), and MEP (L) in the BM were evaluated after 2.5 weeks after drug treatment. Error bars represent mean ± SEM. *P < .05.
WNT974 in combination with nilotinib significantly reduces leukemic stem and progenitor cells in the BM and spleen of transgenic BCR-ABL mice. (A) BCR-ABL expression was induced in SCL-tTA-BCR-ABL mice by tetracycline withdrawal. BM cells were obtained 3 weeks after induction and transplanted into wild-type FVB/N recipient mice irradiated at 750 cGy (106 cells/mouse; 6-8 mice/group). Three weeks posttransplantation, treatment with vehicle, NIL (50 mg/kg), WNT974 (5 mg/kg), or a combination was initiated. PB WBC counts (B), PB neutrophil counts (C), BM cellularity (D), spleen size (E), absolute number of LTHSC (F), STHSC (G), MPP1 (H), MPP2 (I), CMP (J), GMP (K), and MEP (L) in the BM were evaluated after 2.5 weeks after drug treatment. Error bars represent mean ± SEM. *P < .05.
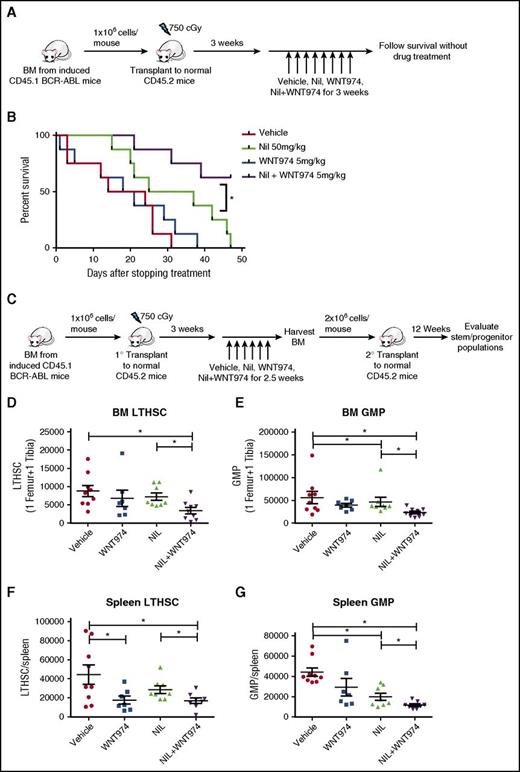
Mice treated with NIL plus WNT974 showed prolonged survival after discontinuation of treatment when compared with mice treated with vehicle (P < .001), WNT974, or NIL alone (vs. WNT974, P < .005; vs. NIL, P < .05) (Figure 5A-B). To assess effects of treatment on self-renewal of CML LSC, we transplanted equal numbers of BM cells (2 × 106 cells/mouse) from drug-treated mice into normal recipient mice (Figure 5C). Although recipient mice did not develop leukocytosis, they showed high levels of donor neutrophil engraftment, consistent with CML engraftment (data not shown). We did not observe reduced engraftment of donor CD45.1+ CML populations in the PB and spleen of recipients of BM cells from combination-treated mice at 12 weeks posttransplantation (supplemental Figure 5G,J). However, CD45.1+ myeloid cells in PB and CD45.1+ cells in BM were significantly reduced in comparison with vehicle and individual drug-treated mice (supplemental Figure 5H and 5I, respectively). There was significantly reduced regeneration of donor LTHSC and GMP in the BM and spleen of secondary recipients of cells from mice treated with WNT974 plus NIL in comparison with vehicle or NIL alone (Figure 5D-G).
WNT974 in combination with nilotinib significantly inhibits regeneration of CML LTHSC after second transplant and prolongs the survival of BCR-ABL mice. (A) BCR-ABL expression was induced in SCL-tTA-BCR-ABL mice by tetracycline withdrawal. BM cells were obtained 3 weeks after induction and transplanted into wild-type FVB/N recipient mice irradiated at 750 cGy (106 cells/mouse; 6-8 mice/group). Three weeks posttransplantation, treatment with vehicle, nilotinib (50 mg/kg), WNT974 (5 mg/kg), or a combination was initiated and continued for 3 weeks. (B) Mice were followed after discontinuation of treatment. (C) BM cells from mice receiving different treatments as described in Figure 4 were pooled and transplanted (2 × 106 cells per mouse; 8-9 mice/group) into wild-type irradiated FVB/N recipient mice for secondary transplantation. Mice were killed after 12 weeks, and the number of BM LTHSC (D), GMP (E), splenic LTHSC (F), and GMP (G) were analyzed. Error bars represent mean ± SEM. *P < .05.
WNT974 in combination with nilotinib significantly inhibits regeneration of CML LTHSC after second transplant and prolongs the survival of BCR-ABL mice. (A) BCR-ABL expression was induced in SCL-tTA-BCR-ABL mice by tetracycline withdrawal. BM cells were obtained 3 weeks after induction and transplanted into wild-type FVB/N recipient mice irradiated at 750 cGy (106 cells/mouse; 6-8 mice/group). Three weeks posttransplantation, treatment with vehicle, nilotinib (50 mg/kg), WNT974 (5 mg/kg), or a combination was initiated and continued for 3 weeks. (B) Mice were followed after discontinuation of treatment. (C) BM cells from mice receiving different treatments as described in Figure 4 were pooled and transplanted (2 × 106 cells per mouse; 8-9 mice/group) into wild-type irradiated FVB/N recipient mice for secondary transplantation. Mice were killed after 12 weeks, and the number of BM LTHSC (D), GMP (E), splenic LTHSC (F), and GMP (G) were analyzed. Error bars represent mean ± SEM. *P < .05.
Healthy FVB/N mice treated for 4 weeks with WNT974, NIL, or the combination of the two maintained stable body weight in comparison with controls (data not shown). No change in WBC or neutrophil counts, or BM and spleen cellularity, was seen in comparison with controls (supplemental Figure 5A-D). There was no significant difference in the number of LTHSC and GMP populations in the BM (supplemental Figure 5E-F). Taken together, these results indicate that WNT974 in combination with NIL inhibits CML, but not normal, stem and progenitor cell growth in vivo.
Enhanced sensitivity to Wnt signaling in CML in comparison with normal stem cells
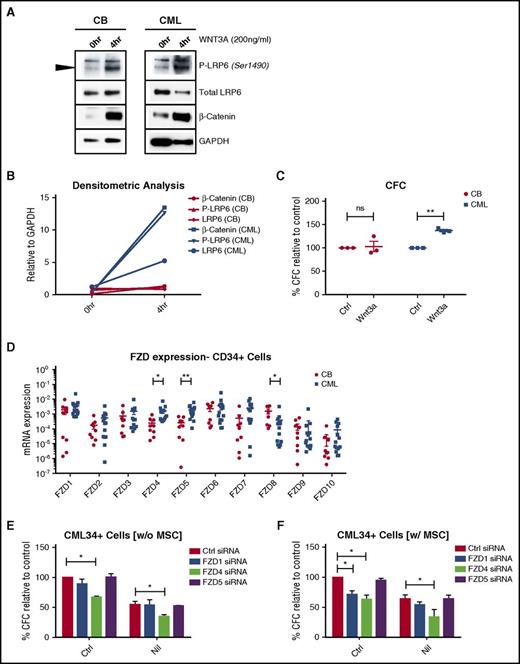
We evaluated the response of CML in comparison with normal CD34+ cells to Wnt exposure. Recombinant Wnt3a (200 ng/mL) resulted in increased phospho-LRP6 (ser1490) and β-catenin levels in CML in comparison with normal CD34+ cells (Figure 6A-B; supplemental Figure 6D-E). Wnt3A-treated CML CD34+ cells formed a significantly increased number of colonies in comparison with normal cells (Figure 6C). RT-qPCR analysis of expression of all 10 FZD genes in CML and normal CD34+ cells revealed significantly increased expression of FZD4 and FZD5, and reduced expression of FZD8, in CML in comparison with normal CD34+ cells (Figure 6D). K562 cells transfected with siRNA against FZD1, FZD4, and FZD5 demonstrated significant inhibition of Wnt reporter activity in comparison with control nontargeting siRNA transfected cells (supplemental Figure 6C). CML CD34+ cells were transfected with FZD1, FZD4, FZD5, and control nontargeting siRNA and target knockdown confirmed using qPCR (supplemental Figure 6A) and Western blotting (supplemental Figure 6B). Knockdown of FZD4 significantly reduced the CFC capacity of CML CD34+ cells both in the presence and absence of MSC, and enhanced inhibition of CML CFC capacity in combination with NIL (Figure 6E-F). These results support an important role for FZD4 in Wnt-mediated regulation of CML progenitor growth and their resistance to TKI treatment.
Enhanced Wnt sensitivity of CML in comparison with normal stem/progenitor cells. (A) Normal and CML CD34+ cells were cultured in recombinant Wnt3A (200 ng/mL), and cells were harvested after 4 h for Western blotting for P-LRP6, LRP6, β-catenin, and GAPDH. (B) Results of densitometry analysis are shown. (C) Normal (CB) and CML CD34+ cells (n = 3) cultured in recombinant Wnt3A (200 ng/mL) were plated in methylcellulose assay, following which colonies were counted after 2 weeks of culture. (D) Results of qPCR analysis was performed for FZD genes in normal and CML CD34+ cells. CML CD34+ cells (n = 3) transfected with control siRNA, FZD1 siRNA, FZD4 siRNA, and FZD5 siRNA were cultured with or without NIL in the absence (E) or presence of MSC (F), for 4 days, following which CFC frequency was analyzed in methylcellulose progenitor assays. Error bars represent mean ± SEM. *P < .05; **P < .01. CB, cord blood.
Enhanced Wnt sensitivity of CML in comparison with normal stem/progenitor cells. (A) Normal and CML CD34+ cells were cultured in recombinant Wnt3A (200 ng/mL), and cells were harvested after 4 h for Western blotting for P-LRP6, LRP6, β-catenin, and GAPDH. (B) Results of densitometry analysis are shown. (C) Normal (CB) and CML CD34+ cells (n = 3) cultured in recombinant Wnt3A (200 ng/mL) were plated in methylcellulose assay, following which colonies were counted after 2 weeks of culture. (D) Results of qPCR analysis was performed for FZD genes in normal and CML CD34+ cells. CML CD34+ cells (n = 3) transfected with control siRNA, FZD1 siRNA, FZD4 siRNA, and FZD5 siRNA were cultured with or without NIL in the absence (E) or presence of MSC (F), for 4 days, following which CFC frequency was analyzed in methylcellulose progenitor assays. Error bars represent mean ± SEM. *P < .05; **P < .01. CB, cord blood.
Discussion
BMM-dependent activation of critical self-renewal signaling pathways may contribute to persistence of BCR-ABL-expressing primitive LSC in CML patients receiving TKI treatment and to recurrence of leukemia on discontinuation of treatment. Wnt signaling is involved in progression of various cancers, via both β-catenin-activating mutations and by paracrine and autocrine Wnt signaling.56 Several strategies for targeting Wnt-β-catenin signaling are being explored. Pharmacological inhibitors of the poly-ADP-ribosyl transferase tankyrase-1 can enhance β-catenin destruction but lack specificity. Small molecules that downregulate β-catenin signaling by binding to cyclic adenosine 5′-monophosphate response element-binding protein are being evaluated in clinical trials. Secretion of all Wnt ligands may be blocked by inhibiting PORCN, a membrane-bound O-acyltransferase required for posttranslational palmitoylation of Wnt ligands.57,58 Palmitoylation of Wnt ligands is required for their secretion and interaction with FZD receptors,59,60 and PORCN inhibitors have been developed as potential anticancer agents.61-64 A PORCN inhibitor, Wnt-C59, blocked progression of mammary tumors in mouse mammary virus tumor (MMTV)-Wnt-1 transgenic mice.62 Another specific and potent PORCN inhibitor, WNT974, significantly inhibits the progression of Wnt-driven tumors in vivo.36 WNT974 inhibited in vitro and in vivo growth of pancreatic cancer cells carrying inactivating mutations of RNF43. These promising results have led to clinical trials of WNT974 in patients with malignancies dependent on Wnt ligands (NCT01351103).
We show here that WNT974 inhibits autocrine and paracrine Wnt signaling in CML progenitors in the context of the BMM. Combined WNT974 and TKI treatment increased inhibition of CML stem/progenitor cells in comparison with TKI alone. WNT974 and TKI treatment was more effective in the context of MSC, supporting the importance of paracrine over autocrine Wnt signaling in CML stem/progenitor cell growth. These drugs had an antiproliferative effect but minimal effect on apoptosis. Wnt inhibition affected both stem and progenitor cells, with the most striking effects seen in the in vivo setting, where combined WNT974 and TKI treatment significantly reduced CML LTHSC frequency, enhanced survival of mice after discontinuation of treatment, and reduced CML LTHSC regeneration after secondary transplantation. In contrast, a recent report indicated that BM mesenchymal cell lines did not activate β-catenin signaling in CML progenitors. However, differences may be related to the use of primary BM MSC in the current study.58 The potent effect of WNT974 on CML stem/progenitor cells in vivo further supports a role for BMM-derived Wnts in their maintenance.
Our results indicate that CML LSC show enhanced response to exogenous Wnt signaling, related at least in part to upregulated expression of FZD4 receptors in CML cells. Inhibition of FZD4 expression reduced Wnt signaling and decreased the colony-forming ability of CML progenitors and enhanced CML progenitor inhibition in combination with NIL. Another recent report also found increased FZD4 and FZD7 expression in CML in comparison with normal CD34+ cells.65 FZD4 upregulation and enhanced Wnt signaling have also been observed in acute myeloid leukemia with the Fms-like tyrosine kinase 3-internal tandem duplication mutation.59,60 FZD4 is also a mediator of erythroblast transformation-specific-related gene-induced Wnt signaling in human prostate cancer cells, and of stemness and invasiveness of glioma cells.66,67 The mechanisms underlying FZD4 upregulation in leukemia cells warrant further investigation.
The combination of WNT974 and TKI did not significantly inhibit normal HSC, suggesting the presence of a therapeutic window that can be exploited in a clinical setting. This is consistent with previous studies indicating a critical role of β-catenin in embryonic but not adult HSC.68 In addition, a recent report by Kabiri et al indicated that adult hematopoiesis was unaffected upon systemic and targeted deletion of PORCN in mice,69 suggesting that autocrine and paracrine Wnts may be dispensable for normal adult hematopoiesis.
About half of CML CP patients who maintain undetectable minimal residual disease can discontinue TKI treatment without disease recurrence. However, most CML patients require continued TKI treatment to prevent relapse, with associated risks of noncompliance, toxicity, and considerable cost. There is a pressing need for approaches to achieve enhanced LSC targeting. Strategies targeting LSC may also reduce more mature downstream progeny. Targeted pathways may also be shared between LSC and progenitors. Multiple approaches to targeting CML LSC are being evaluated, but a single effective and nontoxic approach has been elusive.70 The Wnt pathway is an attractive target because of its important role in regulating CML stem cells and its interaction with other potential targets in CML LSC, such as c-Myc. Our studies showing that PORCN inhibition effectively inhibits Wnt signaling and enhances targeting of CML stem/progenitor cells, without toxicity to normal hematopoiesis, support further evaluation of this approach to targeting residual LSC and enhance TKI discontinuation in CP CML patients.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank StemCyte for cord blood samples. The authors thank Linda Seymour and Vincent Paterno for sample acquisition and are grateful for the assistance of the analytical cytometry core at City of Hope.
This work was supported by National Institutes of Health, National Cancer Institute grant R01CA172447.
Authorship
Contribution: P.A. designed, planned, and conducted experiments, analyzed data, and wrote the manuscript; B.Z., Y.H., A.C., and L.L. conducted the experiments; F.M.M. conducted the experiments; Y.W. designed the experiments and edited the manuscript; M.E.M. provided reagents and edited the manuscript; R.B. designed the experiments, analyzed data, and wrote the manuscript.
Conflict-of-interest disclosure: Y.W. and M.E.M. are employees of Novartis. The remaining authors declare no competing financial interests.
Correspondence: Ravi Bhatia, Division of Hematology and Oncology, Department of Medicine, University of Alabama at Birmingham, 1802 6th Ave S, North Pavilion, Room 2555C, Birmingham, AL 35294-3300; e-mail: rbhatia@uabmc.edu.