Abstract
Cryoglobulinemia is a distinct entity characterized by the presence of cryoglobulins in the serum. Cryoglobulins differ in their composition, which has an impact on the clinical presentation and the underlying disease that triggers cryoglobulin formation. Cryoglobulinemia is categorized into two main subgroups: type I, which is seen exclusively in clonal hematologic diseases, and type II/III, which is called mixed cryoglobulinemia and is seen in hepatitis C virus infection and systemic diseases such as B-cell lineage hematologic malignancies and connective tissue disorders. Clinical presentation is broad and varies between types but includes arthralgia, purpura, skin ulcers, glomerulonephritis, and peripheral neuropathy. Life-threatening manifestations can develop in a small proportion of patients. A full evaluation for the underlying cause is required, because each type requires a different kind of treatment, which should be tailored on the basis of disease severity, underlying disease, and prior therapies. Relapses can be frequent and can result in significant morbidity and cumulative organ impairment. We explore the spectrum of this heterogeneous disease by discussing the disease characteristics of 5 different patients.
Medscape Continuing Medical Education online
This activity has been planned and implemented through the joint providership of Medscape, LLC and the American Society of Hematology.
Medscape, LLC is accredited by the Accreditation Council for Continuing Medical Education (ACCME), the Accreditation Council for Pharmacy Education (ACPE), and the American Nurses Credentialing Center (ANCC), to provide continuing education for the healthcare team.
Medscape, LLC designates this Journal-based CME activity for a maximum of 1.00 AMA PRA Category 1 Credit(s)™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
All other clinicians completing this activity will be issued a certificate of participation. To participate in this journal CME activity: (1) review the learning objectives and author disclosures; (2) study the education content; (3) take the post-test with a 75% minimum passing score and complete the evaluation at http://www.medscape.org/journal/blood; and (4) view/print certificate. For CME questions, see page 396.
Disclosures
Editor Nancy Berliner received grants for clinical research from Novartis. Laurie Barclay, freelance writer and reviewer, Medscape, LLC, owns stock, stock options, or bonds from Pfizer. Author Morie A. Gertz served as an advisor or consultant for Celgene, Millennium Pharmaceuticals, Onyx Pharmaceuticals, Novartis, GlaxoSmithKline, Prothena, Ionis Pharmaceuticals, and Amgen. The remaining authors declare no competing financial interests.
Learning objectives
Upon completion of this activity, participants will be able to:
Determine classification of cryoglobulinemia into types I, II, and III, based on a review with illustrative cases.
Assess clinical manifestations and management of type I cryoglobulinemia.
Assess clinical manifestations and management of mixed cryoglobulinemia.
Release date: January 19, 2017; Expiration date: January 19, 2018
Introduction
Cryoglobulinemia is a clinical disorder characterized by the presence of cryoglobulins in the serum. Cryoglobulins are immunoglobulins that precipitate in vitro at temperatures below normal body temperature (<37°C) and redissolve upon rewarming. This observation was first reported in 1933 in a patient with multiple myeloma.1 The term “cryoglobulin” was coined in 1947, and at that time, cryoglobulins were found to be globulins that appeared in various diseases.2 Cryoglobulinemia may be asymptomatic without end-organ damage and is sometimes discovered as an incidental laboratory finding. The term cryoglobulinemic vasculitis is often used to distinguish the asymptomatic presence of cryoglobulins from cryoglobulins that cause end-organ damage by precipitating in small- to medium-size blood vessels.
Classification of cryoglobulinemia is based on a system developed more than 40 years ago. It has the advantage of correlating with pathogenicity and clinical manifestations, which differ among types.3 In type I cryoglobulinemia, the cryoglobulins are monoclonal immunoglobulins (Ig’s), usually of the IgG or IgM isotypes and rarely IgA or free immunoglobulin light chains.4 Type I cryoglobulinemia develops in the setting of protein-secreting monoclonal gammopathies. Monoclonal gammopathy of undetermined significance (MGUS) is seen in ∼40% of patients, and the remaining 60% have an overt malignancy of B-cell lineage (eg, multiple myeloma [MM], Waldenström macroglobulinemia [WM], or chronic lymphocytic leukemia [CLL]).5,6 Type I cryoglobulin levels do not differ among patients with MGUS and those with B-cell malignancies.6 Therefore, the qualitative characteristics of the cryoglobulins play a greater role in disease pathogenesis than the cryoglobulin burden.
In type II cryoglobulinemia, the cryoglobulins are composed of a mix of monoclonal IgM with rheumatoid factor (RF) activity and polyclonal IgG. This is usually associated with hepatitis C virus (HCV) infection in up to 90% of patients.7 There are geographic variations, and higher rates of HCV infection are seen in the Mediterranean region. Other causes of type II cryoglobulinemia include other infections (mainly HIV and hepatitis B virus [HBV]), connective tissue diseases (CTDs), and lymphoproliferative disorders. Approximately 10% of patients have no identifiable cause (termed essential mixed cryoglobulinemia).8 Type III is characterized by polyclonal IgM with RF activity and polyclonal IgG. This type is seen in CTDs or secondary to infection (mainly HCV). Overall, cryoglobulins in mixed cryoglobulinemia result from a B-cell lymphoproliferative process in the setting of persistent immune activation triggered by chronic infection, autoimmune disease, or an unknown cause.
Pathogenesis
Cryoprecipitation
The process of cryoprecipitation is not entirely understood and probably differs between type I and type II/III. In type I, the monoclonal component undergoes crystallization and aggregation, which is dependent on temperature and concentration.9 Although the definition of cryoglobulins is precipitation at cold temperatures, this process can occur at room temperature at high cryoglobulin concentrations.2 This probably explains why distal extremities (lower temperatures) and kidneys (increase in concentration as a result of ultrafiltration) are major sites of pathology. In type II/III cryoglobulinemia, cryoprecipitation takes place in the setting of immune complex formation between polyclonal IgG and IgM with RF activity and complement fixation. Cryoprecipitation cannot be induced by the IgM or IgG components separately and requires specific antigen-avidity IgG molecules.10 This underscores the unique properties of cryoglobulins and explains the high incidence of HCV as a cause.
Tissue injury
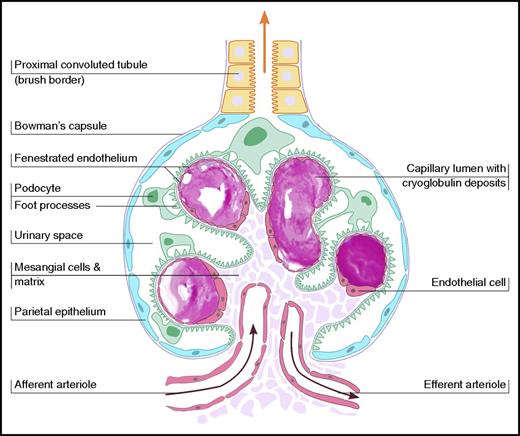
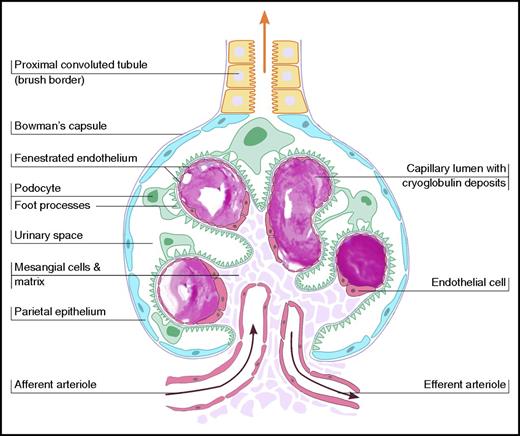
The mechanism of cryoglobulin pathogenicity is best described for HCV-associated cryoglobulinemia.11 HCV drives a clonal B-cell expansion, which secretes a monoclonal IgM that binds to polyclonal IgG molecules, which defines a RF. The IgM interacts with anti-HCV IgG to form immune complexes. These immune complexes bind via a C1q to C1q receptors receptor on endothelial cells, which promotes inflammatory cell recruitment and produces vasculitis. In contrast, the underlying pathogenesis of cryoglobulins in type I is small blood vessel occlusion with minimal inflammatory response (Figure 1).
Schematic illustration of vascular occlusion in type I cryoglobulinemia, demonstrating glomerular intracapillary thrombi.
Schematic illustration of vascular occlusion in type I cryoglobulinemia, demonstrating glomerular intracapillary thrombi.
Diagnosis and laboratory evaluation
The diagnosis of cryoglobulinemia is based on typical clinical manifestations in the presence of cryoglobulins in the serum. False-negative results may occur as a result of improper sample handling. Samples should be transferred and centrifuged at 37°C to avoid precipitation before serum extraction. In type I, precipitation at 1 to 4°C usually occurs within hours; samples should be stored for 7 days because precipitation can be delayed in the mixed types. If the test is negative for cryoglobulin and the index of suspicion remains high, it is appropriate to repeat the test after contacting the laboratory to ensure that the sample has been handled appropriately. At least 5 mL of sample should be obtained to increase diagnostic yield because trace levels of cryoglobulins can be clinically significant.
When cryoglobulins are detected, measurement of the cryocrit (ie, the relative volume of the precipitate as a percentage of the total serum volume) should be reported if possible. The cryocrit level is higher in type I and the lowest in type III12 (can be more than 50% in type I and is usually less than 5% in type II/III). Correlation between the cryocrit and disease manifestations is poor,13,14 although higher cryocrit has been reported to increase the likelihood of symptomatic disease.8 The cryocrit was prognostic in a few studies.15,16 Overall, cryocrit use should be reserved for diagnosis because there is a poor correlation between the cryocrit and the response to treatment.
After cryoprecipitation, the precipitate is washed in a cold buffer and dissolved in a warm solution; electrophoresis and immunofixation are then performed to type the cryoglobulins. Immunofixation may not be feasible if the concentration of cryoglobulins is low.
RF and complement studies (C3, C4, CH50) are part of the evaluation. RF is increased and complement (mainly C4) is decreased in the mixed types but can also be abnormal in type I.6,17 These serologic abnormalities have led to a misdiagnosis of rheumatoid vasculitis because of failure to consider cryoglobulinemia. These parameters do not correlate well with disease activity but can be used to monitor the response to therapy.18 Comparison of clinical manifestations and laboratory findings between type I and mixed cryoglobulinemia is presented in Table 1.
Cryoglobulinemia and hematologic diseases
Hematologic cancers represent less than one quarter of all cryoglobulinemia cases. Type I cryoglobulinemia occurs mainly in B-cell malignancies with predominant protein secretion, including WM, MM, CLL, and the premalignant state MGUS. In contrast, type II cryoglobulinemia is seen more often in patients with WM or other B-cell lymphomas.
Clinical manifestation
Most patients with circulating cryoglobulins remain asymptomatic and do not require immediate intervention. Signs and symptoms depend on the cryoglobulin type.
Case 1: type I cryoglobulinemia
A 40-year-old previously healthy man was seen in the emergency room for intractable epistaxis. He reported having a week of intermittent bleeding that was self-limiting but then became uncontrolled. The patient also reported several months of increasing fatigue and spontaneous bruises on his lower extremities. On examination, the patient appeared pale with active bleeding from both nostrils without an obvious focus. Livedo reticularis in both lower extremities and confluent hematomas were also noted. Laboratory evaluation revealed hemoglobin of 7.8 g/dL, normal platelet count, and normal coagulation studies. Serum total protein was increased and led to identification of an IgM-κ monoclonal protein of 5.7 g/dL with cryoglobulins precipitated at 4°C after several hours. Serum viscosity was increased to 4.8 centipoise, and funduscopic examination revealed asymptomatic retinal bleeding. Urgent plasma exchange was initiated, and the patient experienced symptom relief after 2 exchanges.
Questions
What are the hallmark manifestations of type I cryoglobulinemia?
How should a patient with type I cryoglobulinemia be managed?
Clinical manifestations of type I cryoglobulinemia
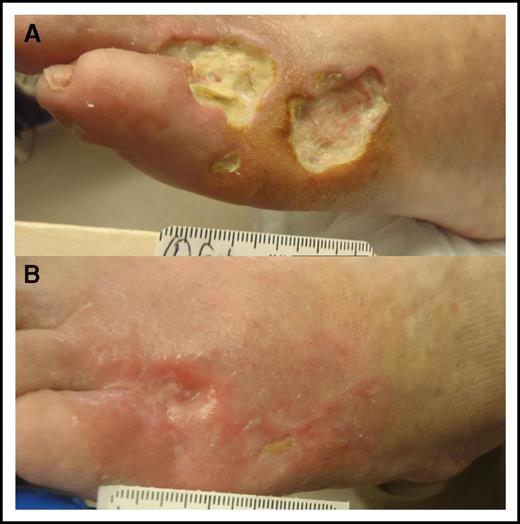
Signs and symptoms are generally associated with vascular occlusion by the cryoprecipitate, although small-vessel vasculitis features may also be seen (eg, purpura, glomerulonephritis, neuropathy). Two studies (64 patients each) reported on the clinical manifestations in type I cryoglobulinemia.5,6 Skin manifestations were the most common and were observed in 69% to 86% of patients. These manifestations include purpura, livedo reticularis, Raynaud's phenomenon, acrocyanosis, skin necrosis, ulcers and, infrequently, digital gangrene. Cutaneous manifestations are often confined to acral sites (distal limbs, nose, and ears) and can be triggered by cold exposure. Skin necrosis (Figure 2) is evident in approximately one third of patients and is mainly seen in the lower extremities in the supramalleolar areas. These ulcers do not heal easily because of impaired blood supply, they put patients at significant risk for sepsis, and at times require amputation. Extracutaneous disease includes peripheral neuropathy in 19% to 44% of patients, arthralgia in 28%, and renal disease in approximately 30%. Painful peripheral neuropathy is usually confined to the lower extremities with predominant sensory impairment, but motor involvement can also be seen. Renal involvement presents as proteinuria, microscopic hematuria, increased creatinine, and/or hypertension and is more common in IgG than IgM cryoglobulinemia.5 Most renal biopsies show membranoproliferative glomerulonephritis (MPGN) with immune cell infiltration, glomerular thrombi, and microtubular deposits composed of the cryoglobulin aggregates (Figure 3).
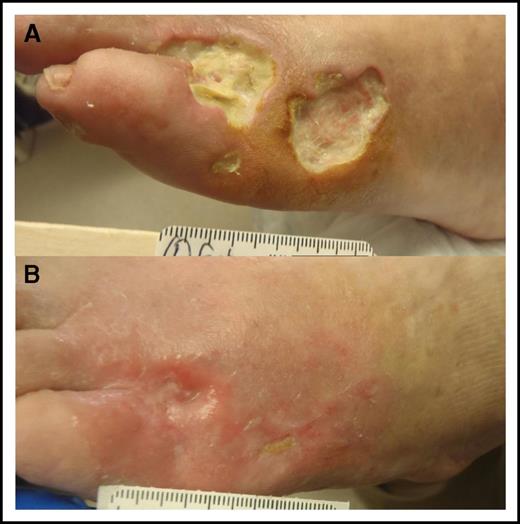
Skin necrosis in cryoglobulinemia. (A) Three skin ulcers at the anterolateral dorsum of the left foot in a patient with newly diagnosed WM-associated mixed cryoglobulinemia. Note that the 2 large ulcers are full skin thickness with minimal surrounding inflammatory response and no tendon or bone exposure. Prophylactic antibiotics were initiated to prevent serious infection. (B) The ulcers healed within 3 months from initiation of definitive therapy for WM.
Skin necrosis in cryoglobulinemia. (A) Three skin ulcers at the anterolateral dorsum of the left foot in a patient with newly diagnosed WM-associated mixed cryoglobulinemia. Note that the 2 large ulcers are full skin thickness with minimal surrounding inflammatory response and no tendon or bone exposure. Prophylactic antibiotics were initiated to prevent serious infection. (B) The ulcers healed within 3 months from initiation of definitive therapy for WM.
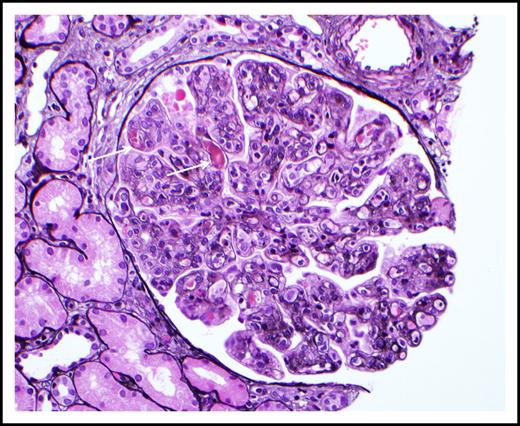
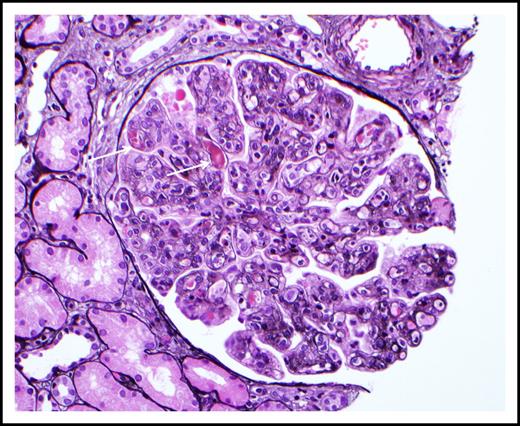
Cryoglobulinemic glomerulonephritis. The glomerulus shows global occlusion of peripheral capillary lumina by numerous infiltrating monocytes. Segmental intracapillary pink-staining immune thrombi (arrows), likely representing cryoglobulin deposits, are seen. There is widespread duplication of the glomerular basement membrane with associated cellular interposition (silver stain). Original magnification ×400. Photo courtesy of Samih Nasr, Mayo Clinic, Rochester, MN.
Cryoglobulinemic glomerulonephritis. The glomerulus shows global occlusion of peripheral capillary lumina by numerous infiltrating monocytes. Segmental intracapillary pink-staining immune thrombi (arrows), likely representing cryoglobulin deposits, are seen. There is widespread duplication of the glomerular basement membrane with associated cellular interposition (silver stain). Original magnification ×400. Photo courtesy of Samih Nasr, Mayo Clinic, Rochester, MN.
When the cryocrit is high, hyperviscosity syndrome may ensue. Symptoms suggestive of hyperviscosity syndrome include oronasal bleeding, blurred vision, sudden deafness, headache, confusion, and heart failure as a result of plasma volume expansion. Measurement of serum viscosity may be helpful in establishing the diagnosis because symptoms of hyperviscosity rarely appear when the viscosity is below 4.0 centipoise (normal level, ≤1.5 centipoise).
Management of type I cryoglobulinemia
Treatment is reserved for symptomatic disease and is directed against the underlying disorder. Complete hematologic evaluation includes bone marrow aspiration and/or biopsy and appropriate imaging studies. High-grade evidence regarding the appropriate approach to treatment for the underlying disease is limited because the incidence is low. Thus, recommendations are mostly expert opinion, based on the best available data. In patients with MM, treatment options follow MM consensus recommendations. In patients with renal failure, a bortezomib-based regimen is warranted, whereas in patients with neuropathy, lenalidomide-based treatment is a reasonable first choice. Before using autologous stem cell transplant, possible organ damage caused by the cryoglobulinemia should be considered. In patients with WM-associated cryoglobulinemia, the use of bortezomib as primary therapy is recommended by the International Workshops on Waldenström’s Macroglobulinemia.19 Rituximab (monoclonal anti-CD20 antibody) as an initial therapy may cause IgM flare and development of hyperviscosity20 ; rituximab can be incorporated after an initial response to treatment has been achieved. Plasma exchange can be used to prevent IgM flare in patients treated with rituximab who have high levels of IgM (above 4 g/dL). Ibrutinib is emerging as an active agent in the management of WM,21 and although no data are available on its use in WM-associated cryoglobulinemia, this therapeutic option may be a legitimate consideration while waiting for clinical data to emerge. In patients with symptomatic hyperviscosity, plasma exchange is warranted but should always parallel definitive cytoreductive therapy. Patients with MGUS have a lower tumor burden, and lower-intensity treatment can initially be attempted. Among 23 patients with MGUS-related type I cryoglobulinemia, initial therapy consisted of single-agent prednisone at a median dose of 60 mg/day.6 Response, defined by improvement in baseline clinical manifestations, was seen in 74% of patients, although half the responders relapsed and required a second line of therapy. Rituximab should be limited to MGUS patients with IgM isotype or when a CD20-positive B-cell clone is detected.5
It is imperative to educate patients on avoiding exposure to the cold by wearing gloves when using the freezer or refrigerator, wearing warm clothes in air-conditioned facilities, and relocating to a warmer climate during the winter months. Foot and leg care is important to prevent wound complications. Diabetic foot care guidelines can be followed for this purpose (eg, checking the feet every day, wearing shoes and socks at all times, and trimming toenails gently).
Case 2: noninfectious mixed cryoglobulinemia
A 55-year-old previously healthy man first noticed decreased tolerance for exercise during a 500-mile bike ride. Subsequently, lower extremity edema developed and 2 skin ulcers were noted over the left anterolateral aspect of the dorsal foot (Figure 2A). Creatinine was 1.4 mg/dL, and 2.2 g of protein (predominantly albumin) was seen in a 24-hour urine collection. A renal biopsy demonstrated MPGN. Immunofluorescence staining was positive for IgM, κ light chain and C3. Congo red stain was negative for amyloid deposits. Serum electrophoresis and immunofixation showed the presence of an IgM-κ band as well as a cryocrit of 9%. Serology was negative for double-stranded DNA and positive for RF activity (915 IU/mL [normal, <15 IU/mL]). Complement tests demonstrated reduced total hemolytic complement assay (CH50), markedly reduced C4 (<2 mg/dL [normal, 14-40 mg/dL]), and a slight reduction in C3 (67 mg/dL [normal, 75-175 mg/dL]). HBV and HCV panels were negative. Bone marrow examination revealed 25% infiltration by a mix of κ-restricted CD20-positive lymphoid cells, lymphoplasmacytic cells, and plasma cells. The diagnosis was WM-associated mixed cryoglobulinemia, and the patient was treated with bendamustine-rituximab for 6 cycles. He achieved a very good partial response to treatment, the proteinuria fully resolved, and the foot ulcers healed (Figure 2B).
Questions
What are the clinical manifestations of mixed cryoglobulinemia?
What are the treatment options for noninfectious mixed cryoglobulinemia?
Clinical manifestations of mixed cryoglobulinemia
Mixed cryoglobulinemia, compared with type I cryoglobulinemia, is more often associated with constitutional symptoms such as fever, weakness, myalgia/arthralgia, and anorexia reflecting an immune complex disorder. The disease course is often chronic with flares followed by symptom resolution. The triad of purpura, arthralgia, and weakness (Meltzer’s triad)22 is well known but is seen in only one third of patients.12 Purpura is the most common manifestation, is seen in up to 90% of patients, and is a key feature of the diagnosis.10 It can be transient or persistent. Cold exposure can trigger a purpura flare, but more common triggers are protracted standing, exercise, depilation, drugs, and infections.3,23 Purpura usually involves the legs (gravitational) but can extend to the torso and upper extremities, can be isolated or can accompany a more widespread vasculitis, including livedo reticularis and skin ulcers. Histopathologic examination of the purpuric lesions reveals leukocytoclastic vasculitis with fibrinoid necrosis. Arthralgia is common in elbows, wrists, and knees, whereas arthritis with overt joint effusion is seen only in a small proportion of patients.24 Renal disease and neuropathy are similar to those seen in type I, although type I is more likely to demonstrate small vessel obstruction by cryoglobulin aggregates.25 Involvement of organs other than the kidneys is rare but when present, it almost exclusively points to mixed cryoglobulinemia. Dyspnea and dry cough can be signs of pulmonary involvement in the form of fibrosis or infiltrates that result from organizing pneumonia or alveolar hemorrhage.26,27 Gastrointestinal (GI) involvement should be considered in patients with cryoglobulinemia who present with acute abdominal pain and/or GI bleeding as a sign of intestinal vasculitis and ischemia with risk of perforation.28 Central nervous system manifestations of cryoglobulinemia are usually in the form of ischemic stroke, rarely as confusion. Heart involvement is rare, with the main manifestation being myocardial ischemia and dilated cardiomyopathy.29 In a cohort of 209 patients with cryoglobulinemia, 14% had life-threatening involvement defined by acute renal failure, intestinal ischemia, pulmonary hemorrhage, and/or central nervous system involvement.30 A life-threatening event was the initial presentation in 59% of patients. Compared with patients with more subtle symptoms, life-threatening involvement was associated with a higher frequency of fever, type II cryoglobulinemia, higher cryocrit, and a low C3. Mortality was 100% in patients with GI involvement and pulmonary hemorrhage.
Treatment options for noninfectious mixed cryoglobulinemia
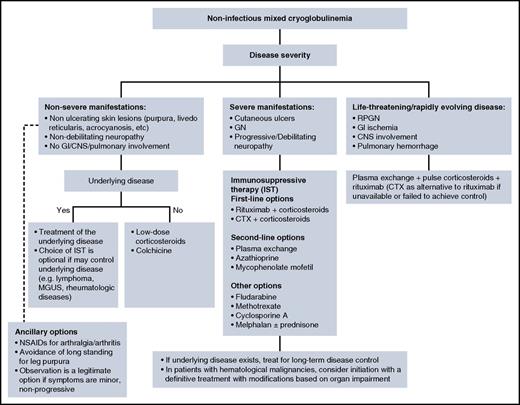
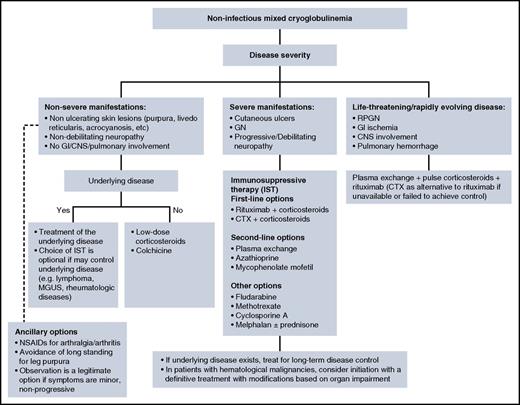
Given the rarity of non-HCV mixed cryoglobulinemia, it is challenging to provide evidence-based treatment recommendations. Moreover, treatment of noninfectious cryoglobulinemia is provided by both hematologists and rheumatologists, and treatment philosophies may differ between disciplines. When a treatment plan is being considered, there are several general principles to follow, as shown in Figure 4. Treatment of the underlying disease is not always feasible when a cause has not been identified. In patients with overt hematologic malignancy, we prefer to treat the malignancy and modify the treatment according to disease extent and organ dysfunction. Patients with severe or life-threatening manifestations need urgent intervention to suppress immune complex formation. This is accomplished with immunosuppressive therapy (IST), which is used in other systemic vasculitis. IST is primarily based on high-dose corticosteroids, cyclophosphamide, rituximab, and plasmapheresis.
Management algorithm for non-infectious mixed cryoglobulinemia. CNS, central nervous system; CTX, cyclophosphamide; GN, glomerulonephritis; NSAIDS, nonsteroidal anti-inflammatory drugs; RPGN, rapidly progressive glomerulonephritis.
Management algorithm for non-infectious mixed cryoglobulinemia. CNS, central nervous system; CTX, cyclophosphamide; GN, glomerulonephritis; NSAIDS, nonsteroidal anti-inflammatory drugs; RPGN, rapidly progressive glomerulonephritis.
IST is intended to halt end-organ damage, and once the treatment goal has been achieved, the dose should be tapered to the lowest possible dose and should be discontinued, if possible. Rituximab, as a B-cell depletion agent, constitutes a major element in the current paradigm of the immunosuppressive approach. Four weekly doses of 375 mg/m2 are usually given.31 The largest data set on the use of rituximab in noninfectious mixed cryoglobulinemia comes from a Cryovac multicenter survey that reported on 242 patients. The use of rituximab in combination with corticosteroids achieved the greatest benefit in terms of clinical response, renal response, and immunologic response (ie, >50% decrease in the baseline cryoglobulin level and/or a >50% increase in serum C4).32 This combination was more efficacious than corticosteroids alone or corticosteroids in combination with an alkylator. However, patients treated with rituximab and corticosteroids experienced a ninefold increase in severe infections (primarily when the prednisone dose was >50 mg/d), with no adverse effect on survival. Alkylators, mainly cyclophosphamide (2 mg/kg orally once per day or a once-per-month intravenous pulse of 500-750 mg/m2), remain a valid option, because data from randomized controlled trials comparing rituximab to alkylators in the noninfectious setting are limited.33 The fludarabine-cyclophosphamide-rituximab regimen was shown to be active in 7 patients with refractory cryoglobulinemia associated with lymphoma.34 In life- or organ-threatening events, cyclophosphamide can be used in conjunction with pulse corticosteroids (intravenous methylprednisolone 500-1000 mg for 3 days followed by a prednisone taper). A response to plasma exchange in mixed cryoglobulinemia is seen in 70% to 80% of patients,35 and plasma exchange is a rational therapeutic option with severe disease manifestations (MPGN, leg ulcers) or in the case of life-threatening events such as pulmonary hemorrhage or intestinal vasculitis. The exchange solution should be warmed to body temperature to avoid cryoglobulin precipitation.36
If a response is achieved, we usually schedule close follow-up visits once every 3 months, because relapses frequently occur. Follow-up visits can be scheduled for two times per year if remission is maintained for more than 2 years. During follow-up visits, detailed interval history and careful physical examination are the key components in assessing remission status. In addition, our routine laboratory evaluation at each visit includes complete blood count, chemistry panel, cryoglobulin measurement (and monoclonal protein measurement if it was present at diagnosis), RF and complement measurements if they were abnormal at presentation, urinalysis, and 24-hour urine collection for proteinuria.
It is also imperative to assess for infections in this population and adhere to recommended vaccinations for the immunocompromised population. Monitoring for malignancies, particularly hepatocellular carcinoma in patients with chronic viral hepatitis and hematologic malignancies in patients with MGUS or CTDs, should be performed.37-39
Case 3: relapsed cryoglobulinemia
A 62-year-old woman was diagnosed with type II non-HCV cryoglobulinemia affecting the kidneys and skin. A small clone of CD20-positive κ-restricted lymphocytes was seen in a bone marrow biopsy. She was successfully treated with rituximab and prednisone and achieved complete resolution of her symptoms with reduction in the cryocrit >80%. She relapsed after 2 years off treatment, with an increase in urine protein (>1 g/day) and leg purpura.
Questions
What is the natural course of mixed cryoglobulinemia?
What are the treatment options for disease recurrence and/or progression?
Little data are available on the rate of progression or relapse over the disease course. In a study of 47 treated patients with mixed cryoglobulinemia, an average of 3.8 treatments per patient were given over a median follow-up of 3.2 years,13 emphasizing the relapsing nature of the disease. Moreover, many patients have underlying indolent clonal hematologic disease (eg, indolent lymphoma) or CTD, which have frequent relapses and may contribute to the relapsing-remitting nature of mixed cryoglobulinemia. Noninfectious mixed cryoglobulinemia is associated with a 10-year survival of 79%, similar to the survival among patients with mixed cryoglobulinemia associated with HCV (10-year survival, 75%).40 The leading cause of death in this population is severe infections, which account for half the deaths. More than 80% of the infection-related deaths occurred during the induction phase of treatment at a median of 50 days from initiation. Nineteen percent of the deaths were the result of vasculitis flares, predominantly renal. In comparison, the leading causes of death in HCV-associated cryoglobulinemia are infections (35%), end-stage liver disease (30%), and end-organ damage resulting from vasculitis (17%).41
Treatment at progression should follow the outline for initial treatment, with adjustments according to the nature of the progression. If initial treatment was successful with durable response (>1 year), that regimen can be repeated. Otherwise, an alternative regimen would be appropriate. It is also appropriate to re-stage the underlying disease because patients with MGUS or CTD can progress to overt hematologic malignancy, which can have an impact on the choice of treatment.
Case 4: HCV-associated (infectious) mixed cryoglobulinemia
A 55-year-old woman HCV carrier developed weakness and arthralgia over several months. Muscle weakness was accompanied by a painful burning sensation in her lower extremities, which prompted further investigation. On examination, symmetrically distributed palpable purpura were observed in the lower extremities. Muscle strength was 4/5 in the lower extremities and preserved in the upper extremities. Tendon reflexes were absent at the ankles bilaterally and reduced at the knee. Cryoglobulins were detected in the serum with an IgM-κ monoclonal protein and 3% cryocrit. RF activity was increased (765 IU/mL), and C4 was below normal (5.4 mg/dL). Creatinine was 1.3 mg/dL, and urinalysis demonstrated microscopic hematuria and proteinuria (1.5 g/24 hours). HCV polymerase chain reaction demonstrated the presence of viral RNA genotype 1a. The patient initiated treatment with rituximab and prednisone followed by ribavirin and peg-interferon.
Questions
What are the treatment options for infection-associated mixed cryoglobulinemia?
Does rituximab have a role and is it safe in virus-associated mixed cryoglobulinemia?
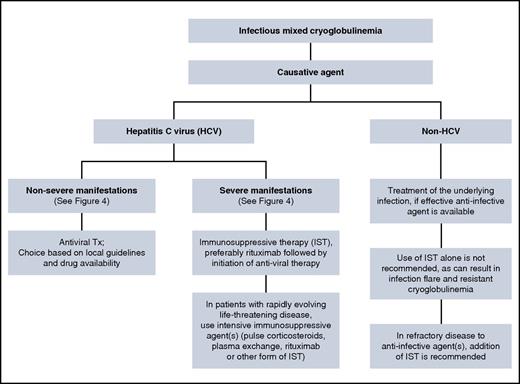
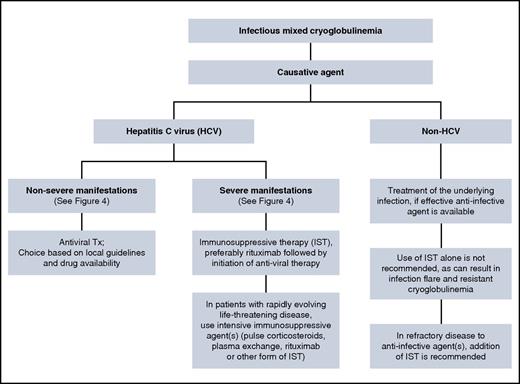
The principles of infectious-associated mixed cryoglobulinemia follow those used for noninfectious mixed cryoglobulinemia (Figure 5). Patients with HCV-associated severe cryoglobulinemia are usually offered IST followed by combination antiviral therapy. Gradual introduction of treatment aims to avoid cumulative toxicities.42 In our patient, glomerulonephritis and sensorimotor neuropathy indicated severe disease, and IST is the preferred initial treatment to halt immune complex–mediated organ damage. The choice of antiviral treatment should be made according to current guidelines,43-46 because the presence of cryoglobulinemia does not have an impact on the choice of antiviral therapy. Direct-acting antiviral agents (DAAs) are more potent than the pegylated interferon-ribavirin combination, are given orally, for a shorter duration with a better safely profile.47 This may be relevant because patients with mixed cryoglobulinemia are often anemic and/or have renal impairment, which complicates the use of ribavirin in these patients, whereas DAAs can safely be given to most patients with renal and hepatic impairment.48 Although clinical improvement can occur even without achieving HCV RNA clearance (sustained virologic response [SVR]), patients who achieved SVR were more likely to have improvement in their disease manifestations.49,50 The presence of cryoglobulinemia (with or without symptoms) in HCV patients was an independent negative predictor for attaining SVR in patients treated with pegylated interferon-ribavirin combination.50 Data on the use of DAAs in mixed cryoglobulinemia are emerging with encouraging results.51-54
Rituximab was shown to be effective in HCV-associated cryoglobulinemia. In a prospective cohort study, the addition of rituximab to pegylated interferon-ribavirin resulted in a shorter time to response and better renal response and cryoglobulin clearance, although the rate and depth of clinical response and the relapse rate were similar between study arms. Two randomized trials evaluated rituximab in mixed cryoglobulinemia, mostly in the setting of HCV. In the first trial, 2 doses of rituximab 1 g/m2 days 1 and 14 were compared with other IST therapies in patients for whom anti-HCV therapy failed or who could not tolerate it. At 12 months, a treatment-free status was more likely in the rituximab arm. Organ improvement was better with rituximab, predominantly for skin and renal involvement.33 The second trial (with a similar design) evaluated rituximab (375 mg/m2, 4 weekly doses) compared with other IST therapies. At 6 months, the remission rate was 83% in the rituximab arm compared with 8% in the control arm.55
Data on the therapeutic approach in non-HCV infectious cryoglobulinemia are limited, given the rarity of this disease. A review of 45 patients with non-HCV infectious cryoglobulinemia suggests that disease management in this case is somewhat different than that outlined for HCV-associated cryoglobulinemia56 (Figure 5). Patients who received appropriate anti-infective therapies (antiviral or antibacterial) were more likely to achieve a sustained response, even in the absence of IST. The use of IST alone (including corticosteroids alone) in these patients resulted in a poor response to therapy. And finally, in refractory cases, the use of targeted anti-infective agents in combination with IST may overcome refractoriness to either agent alone. Concomitant anti-infective treatment in non-HCV cases relies on the fact that rituximab is associated with reactivation of HBV57-59 and HIV60,61 in the untreated state. Therefore, rituximab (as well as other forms of IST) should be given only to patients who are receiving concomitant anti-HBV and/or anti-HIV therapies. In contrast, there is evidence suggesting that rituximab does not have an impact on HCV replication,55,62,63 although there are reports indicating an increase in HCV RNA in the serum after rituximab administration, albeit without adverse clinical consequences.64,65
Case 5: asymptomatic cryoglobulinemia
A 55-year-old woman was diagnosed with CLL after being evaluated for an increased white blood cell count during her annual physical examination. She was asymptomatic without an indication for treatment and was put on observation. Two years after the diagnosis, cryoglobulins were found in her serum. Immunofixation of the cryoglobulins revealed a monoclonal IgG-κ. She had no cryoglobulinemia-related symptoms.
Questions
What is the frequency of cryoglobulinemia without associated symptoms?
When is it appropriate to screen for cryoglobulins?
Cryoglobulins can be detected in the sera of healthy people but usually at a very low concentration.66,67 The prevalence of circulating cryoglobulins in the HCV-infected population is close to 50%,68 but only 15% of people in that group show clinical signs of cryoglobulinemia.69 The prevalence of circulating cryoglobulins in hematologic and rheumatologic diseases is likely underreported. In a study that assessed type I cryoglobulins from a laboratory database, 227 patients had circulating type I cryoglobulins, but only 36 patients (16%) had symptomatic disease.17 Several clinical findings should prompt evaluation for cryoglobulinemia (Table 2).
Summary
In conclusion, cryoglobulinemia is a rare clinical entity. Awareness of its clinical spectrum is important, because diagnosis is facilitated by the demonstration of cryoglobulins in the serum. Treatment is challenging, given the end-organ damage and frequent relapses. Currently all treatment options are designed to either treat the underlying disease or to provide immunosuppression, without any emerging disease-specific interventions. Because cryoglobulinemia is a heterogeneous disease in its manifestations and causation, it is difficult to propose uniform management guidelines, although there are similarities in management approaches, as outlined earlier. There is a lack of good-quality data, especially for the less common cryoglobulinemia subtypes. Among the open questions are the components of treatment and their sequence within each subtype and the duration of treatment that will produce a sustained remission, especially in light of short remissions. Data from large cohorts of patients from nationwide or even multinational registries are a key component in trying to sort out the important principles of therapy.
Authorship
Contribution: E.M., H.M., and M.A.G. wrote the paper.
Conflict-of-interest disclosure: M.A.G. has received honoraria from Celgene, Onyx Pharmaceuticals, Novartis, GlaxoSmithKline, Prothena, Ionis Pharmaceuticals, and Amgen, and has performed as a consultant and received honoraria from Millennium Pharmaceuticals. The remaining authors declare no competing financial interests.
Correspondence: Morie A. Gertz, Division of Hematology, Mayo Clinic, 200 First St SW, Rochester, MN 55905; e-mail: gertz.morie@mayo.edu.