Key Points
Small-molecule inhibition and CRISPR/Cas9 deletion of AHR promote early hematoendothelial cell differentiation from hESCs.
AHR inhibition enhances the differentiation of cNK cells from hESCs whereas AHR hyperactivation supports development of ILC3s.
Abstract
The aryl hydrocarbon receptor (AHR) plays an important physiological role in hematopoiesis. AHR is highly expressed in hematopoietic stem and progenitor cells (HSPCs) and inhibition of AHR results in a marked expansion of human umbilical cord blood–derived HSPCs following cytokine stimulation. It is unknown whether AHR also contributes earlier in human hematopoietic development. To model hematopoiesis, human embryonic stem cells (hESCs) were allowed to differentiate in defined conditions in the presence of the AHR antagonist StemReginin-1 (SR-1) or the AHR agonist 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD). We demonstrate a significant increase in CD34+CD31+ hematoendothelial cells in SR-1–treated hESCs, as well as a twofold expansion of CD34+CD45+ hematopoietic progenitor cells. Hematopoietic progenitor cells were also significantly increased by SR-1 as quantified by standard hematopoietic colony-forming assays. Using a clustered regularly interspaced short palindromic repeats (CRISPR)/CRISPR-associated protein 9 (Cas9)-engineered hESC-RUNX1c-tdTomato reporter cell line with AHR deletion, we further demonstrate a marked enhancement of hematopoietic differentiation relative to wild-type hESCs. We also evaluated whether AHR antagonism could promote innate lymphoid cell differentiation from hESCs. SR-1 increased conventional natural killer (cNK) cell differentiation, whereas TCDD treatment blocked cNK development and supported group 3 innate lymphoid cell (ILC3) differentiation. Collectively, these results demonstrate that AHR regulates early human hematolymphoid cell development and may be targeted to enhance production of specific cell populations derived from human pluripotent stem cells.
Introduction
Human pluripotent stem cells function as an important model system to elucidate basic genetic and cell-signaling mediators of human hematopoietic development.1-3 Previous studies demonstrate development of erythroid,4,5 myeloid,6-9 and lymphoid10-12 cells from human embryonic stem cells (hESCs) and human induced pluripotent stem cells (hiPSCs). However, the molecular regulation of earlier human hematopoietic stem and progenitor cells (HSPCs) from pluripotent stem cells remains less well understood. Functional HSPCs develop during the definitive stage of hematopoiesis directly from specialized hemogenic endothelium in a process known as the endothelial-to-hematopoietic transition (EHT).13,14 Hemogenic endothelium capable of EHT has been identified from hESCs/hiPSCs and can thus be used as a platform to investigate the mechanistic cues supporting human HSPC development.12,15,16
The aryl hydrocarbon receptor (AHR) is a member of the Per/Arnt/Sim family of environment-sensing, basic helix-loop-helix transcriptional regulators that is well known for its ability to mitigate reactive oxygen species due to extracellular stressors. However, there is increasing evidence for an important physiological role of AHR in hematopoiesis.17 AHR messenger RNA (mRNA) and protein are enriched in both murine and human HSPCs, with a significant reduction in expression at the onset of HSPC proliferation.18,19 Ahr knockout mice yield an increased number of bone marrow–derived Lin−Sca+Kit+ HSPCs that are hyperproliferative and have an increased propensity for leukemogenesis.20 This finding has been extended to human HSPCs through directed small-molecule targeting of AHR in CD34+ umbilical cord blood (UCB). Treatment with StemReginin-1 (SR-1), a potent human-specific antagonist of AHR, substantially increases the proportion of engraftable UCB CD34+ cells while also sustaining hematopoietic multipotency.21 This strategy has recently been used in clinical trials that demonstrate dramatic HSPC expansion and an improved time to neutrophil engraftment following transplantation with SR-1–expanded UCB.22
Although these results confirm the integral role of AHR in the maintenance of HSPCs, there are a paucity of studies investigating what function, if any, AHR has in the initial differentiation of hematopoietic cells from mesodermal and endothelial progenitor cells. Here, we use hESCs differentiated in chemically defined conditions to test the hypothesis that AHR regulates early human hematopoietic development at the stage of EHT. We demonstrate that inhibition of AHR using SR-1 or deletion of AHR using clustered regularly interspaced short palindromic repeats (CRISPR)/CRISPR-associated protein 9 (Cas9) leads to increased hematoendothelial and functional hematopoietic progenitor cell differentiation. Additionally, we provide novel evidence that AHR inhibition also improves development of conventional natural killer (cNK) cells from hESCs, whereas AHR hyperactivation supports group 3 innate lymphoid cell (ILC3) differentiation. Collectively, these studies demonstrate that AHR inhibition enhances both early human HSPC and lymphoid development, and this strategy may be useful to improve the quantity and homogeneity of clinically useful hematopoietic cell populations derived from human pluripotent stem cells.
Materials and methods
Hematoendothelial differentiation of hESCs
Single-cell adapted hESCs (H9) and 2 CD34+ umbilical cord blood–derived hiPSCs (UCBiPSC7 and DUB7) were maintained on irradiated mouse embryonic fibroblasts (MEFs) in embryonic stem cell growth media, as previously described.23 hESCs were allowed to differentiate as spin embryoid bodies (EBs) as previously described (Figure 1A).23,24 In brief, hESCs were plated at 3000 cells per 100 μL in a round-bottom 96-well plate using serum-free bovine serum albumin polyvinyl alchohol essential lipid (BPEL) media supplemented with 20 ng/mL bone morphogenetic protein 4 (BMP4), 40 ng/mL stem cell factor (SCF), and 20 ng/mL vascular endothelial growth factor (VEGF) (all obtained from R&D Systems, Minneapolis, MN). Cells were centrifuged to form EBs (defined as day 0) and were incubated for 6 additional days (defined as day 6) to promote mesoderm induction. To differentiate early endothelial and hematopoietic progenitor cells, day 6 EBs were transferred to pregelatinized 24-well plates (∼8-16 EBs per well) with BPEL media (without polyvinyl alcohol) supplemented with 40 ng/mL SCF, 40 ng/mL VEGF, 30 mg/mL thrombopoietin (all obtained from R&D Systems), 30 ng/mL interleukin-3 (IL-3), and 30 ng/mL IL-6 (both PeproTech, Rocky Hill, NJ). To modulate AHR activity, EBs were treated at day 6+0 with dimethyl sulfoxide (DMSO), 1 μM SR-1 (Cellagen Technologies, San Diego, CA), or 10 nM 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) (Sigma-Aldrich, St. Louis, MO). Media was exchanged every 3 days with small-molecule and cytokine supplementation. At indicated time points, nonadherent cell fractions were collected and saved whereas the remaining adherent fractions were treated with 0.05% trypsin containing 2% chicken serum. Adherent cells were combined with the nonadherent fractions for analysis, unless otherwise stated.
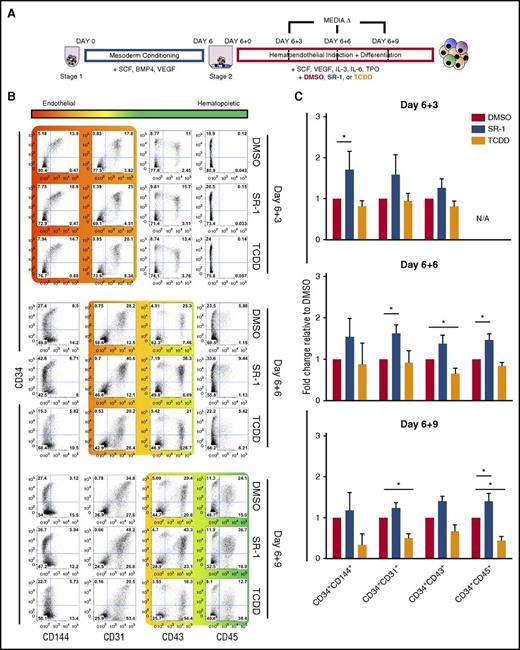
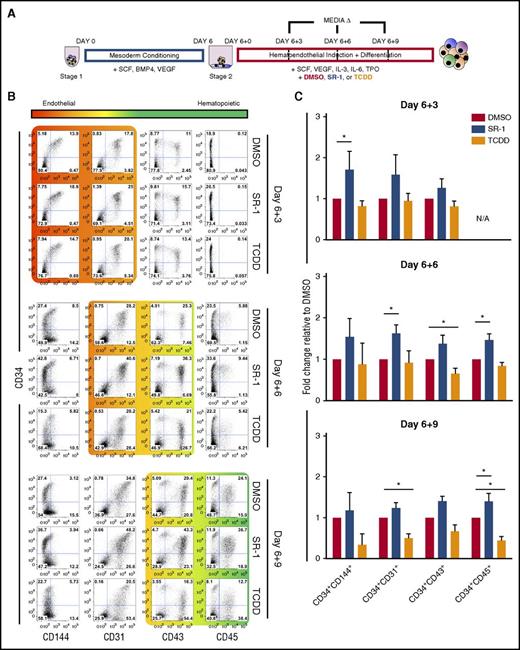
Small-molecule antagonism of AHR enhances early hematoendothelial development from hESCs. (A) Schema of hESC differentiation into early hematoendothelial cells as spin EBs. hESCs are made into spin EBs at day 0 and conditioned into mesoderm lineages for 6 days using defined cytokines (stage 1). At day 6, spin EBs are transferred into hematoendothelial culture media (stage 2) to promote endothelial and hematopoietic cell differentiation. For these studies, cells are treated with either 1 μM SR-1, 10 nM TCDD, or DMSO vehicle control beginning at day 6+0 with media exchanges and/or harvesting performed at day 6+3, day 6+6, and day 6+9. (B) Representative flow cytometry plots of 1 hESC differentiation. Both adherent and nonadherent cell fractions are harvested at day 6+3, day 6+6, and day 6+9 and assessed for endothelial cell (CD34+CD31+, CD34+CD144+) and hematopoietic progenitor cell (CD34+CD43+, CD34+CD45+) phenotype. (C) Fold change of the total percentage of each hematoendothelial phenotype for SR-1– and TCDD-treated hESCs normalized to matched DMSO-treated controls; n = 4-6, error bars represent standard error of the mean (SEM). *P < .05 as compared with DMSO-treated controls by the Student t test. N/A, not applicable due to absence appreciable of CD34+CD45+ populations at day 6+3 time point; TPO, thrombopoietin.
Small-molecule antagonism of AHR enhances early hematoendothelial development from hESCs. (A) Schema of hESC differentiation into early hematoendothelial cells as spin EBs. hESCs are made into spin EBs at day 0 and conditioned into mesoderm lineages for 6 days using defined cytokines (stage 1). At day 6, spin EBs are transferred into hematoendothelial culture media (stage 2) to promote endothelial and hematopoietic cell differentiation. For these studies, cells are treated with either 1 μM SR-1, 10 nM TCDD, or DMSO vehicle control beginning at day 6+0 with media exchanges and/or harvesting performed at day 6+3, day 6+6, and day 6+9. (B) Representative flow cytometry plots of 1 hESC differentiation. Both adherent and nonadherent cell fractions are harvested at day 6+3, day 6+6, and day 6+9 and assessed for endothelial cell (CD34+CD31+, CD34+CD144+) and hematopoietic progenitor cell (CD34+CD43+, CD34+CD45+) phenotype. (C) Fold change of the total percentage of each hematoendothelial phenotype for SR-1– and TCDD-treated hESCs normalized to matched DMSO-treated controls; n = 4-6, error bars represent standard error of the mean (SEM). *P < .05 as compared with DMSO-treated controls by the Student t test. N/A, not applicable due to absence appreciable of CD34+CD45+ populations at day 6+3 time point; TPO, thrombopoietin.
ILC (cNK cell and ILC3) differentiation from spin EBs
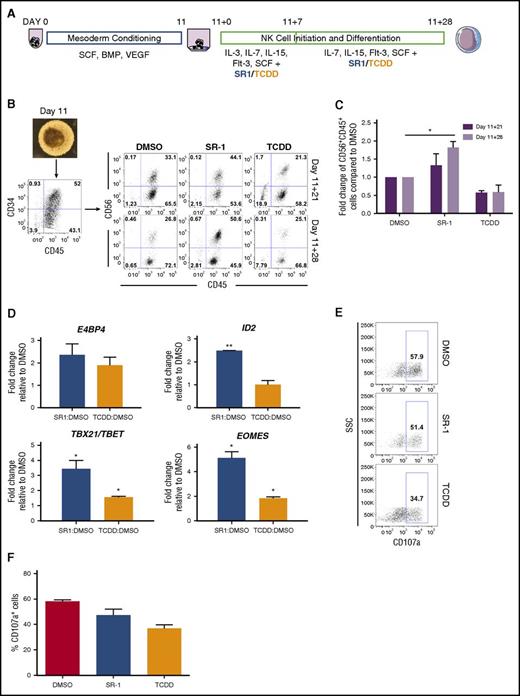
Spin EBs were generated as described in the previous section.10,11,25 Following 11 days of mesoderm conditioning (day 11), spin EBs were collected and analyzed by flow cytometry to assess hematopoietic progenitor cell potential (see “Flow cytometry” for antibodies used) (Figure 4A). Spin EBs yielding >30% CD34+CD45+ cells were transferred onto 24-well plates coated with irradiated OP9-DL1 cells26,27 (now defined as day 11+0). EBs and OP9-DL1 were cocultured in natural killer (NK) differentiation media (NKDM) supplemented initially with SCF, IL-15, IL-7, Flt3-L (all obtained from R&D Systems), and IL-3 (PeproTech) for 1 week; DMSO, SR-1, or TCDD were also added at day 11+0. Every week, a one-half media change with NKDM supplemented with SCF, IL-15, IL-7, Flt3-L, and drugs was performed. hESCs were differentiated for 4 additional weeks (day 11+28) and nonadherent cells were harvested for analysis.
Hematopoietic colony-forming unit assay
Bulk and sorted CD34+CD45+ cells from day 6+5 spin EB nonadherent fractions were collected and resuspended in Iscove modified Dulbecco medium. Fifty thousand cells were seeded in 2 mL of H4436 Methocult (StemCell Technologies, Vancouver, BC, Canada) and plated directly in 35-mm culture dishes (Greiner, Monroe, NC). Plates were incubated for 14 days and subsequently counted and phenotypically scored using standard criteria.9
CRISPR-Cas9 gene editing and hESC transfection
Guide RNA (gRNA) against AHR exon 1 (5′-TCAGATTGTCCCTGGAGGTC-3′) driven by U6 promoter was subcloned into a pCR4-TOPO vector (ThermoFisher Scientific). Single-cell, cell-adapted H9 RUNX1c-tdTomato reporter cell lines previously produced by our group23 were transfected with 1 μg of plasmid DNA, 1 μg of Cas9 mRNA (TriLink Biotechnologies, San Diego, CA), and mCherry fluorescent protein mRNA using the Neon Transfection System (ThermoFisher Scientific) set at 1100 V, 20 ms, 1 pulse. Posttransfection, cells were resuspended in MEF-conditioned media without antibiotics supplemented with 5 μM Y-27632 and seeded onto Matrigel-coated 6-well plates. Ninety-six hours posttransfection, individual mCherry+ colonies were picked onto fresh MEFs for clonal expansion. Genomic DNA was isolated using the DNeasy Blood and Tissue kit (Qiagen) and AHR polymerase chain reaction (PCR) products were generated with high-fidelity AccuPrime Taq DNA Polymerase (ThermoFisher Scientific). PCR products were purified and subcloned into a pCR-TOPO4 vector for sequencing. Cloned products were transformed into One Shot TOP10 competent cells (ThermoFisher Scientific) and were colony sequenced via rolling circle amplification (Sequetech, Mountain View, CA) using M13R primers. On- and off-target effects were assessed using a Surveyor mutation detection kit (IDT Technologies, Coralville, IA) and the following AHR-specific primers: forward, 5′-AGGCAGCTCACCTGTACT-3′; reverse, 5′-CATCTCGCCTTACCAAACTCTAC-3′. Only clones that displayed AHR-specific cleavage products and had AHR exon 1–specific deletions as determined by sequencing were chosen for experiments.
Flow cytometry
The following additional antibodies were used (all anti-human): lymphocyte function-associated antigen 1 (LFA1) (CD11a/CD18)-allophycocyanin (APC)-R700 (BD Biosciences), CD31-APC (eBioscience), CD33-APC (BD Biosciences), CD34-phycoerythrin (PE) Cy7 (BD Biosciences), CD34-APC (BD Biosciences), CD41a-APC (BD Biosciences), CD43-APC (BD Biosciences), CD45-APC (BD Biosciences), CD56-PECy7 (BD Biosciences), CD56-BV421 (BD Biosciences), CD94–peridinin chlorophyll–Cy5.5 (BD Biosciences), CD117-PECy7 (BD Biosciences), CD117-APC (eBiosciences), CD144-APC (eBioscience). Samples were analyzed on either an LSRFortessa or LSRII flow cytometer (BD Biosciences). Gating was set relative to isotype controls of identical fluorophores. Data from flow cytometry were analyzed using FlowJo software (TreeStar, Ashland, OR).
Additional methods
Comprehensive methods regarding, fluorescent-activated cell sorting, CD34+ cell expansion, quantitative real-time PCR (qRT-PCR), immunoblotting, CD107a degranulation assay, and statistical analyses are detailed in the supplemental Methods (available on the Blood Web site).
Results
Small-molecule antagonism of AHR enhances early hematoendothelial differentiation from hESCs
To establish whether AHR mediates development of the earliest human hematopoietic cells, we differentiated hESCs using a 2-stage defined culture system, as previously described (Figure 1A).23,24 We first determined AHR expression in undifferentiated hESCs and hESCs differentiating into hematoendothelial cells under these defined conditions. AHR expression was increased 4.30- ± 1.24-fold in the differentiated cell population at day 6+3 relative to undifferentiated hESCs and became significantly increased at day 6+5 (7.33- ± 1.24-fold; P < .01) (supplemental Figure 1A). We also observed a corresponding increase in the expression of 2 downstream effector targets of AHR signaling, CYP1A1 and CYP1B1.28-33 These data indicate that endogenous AHR activity is upregulated at the onset of hematoendothelial differentiation from hESCs, suggesting that AHR is implicated in early hematopoiesis. We next treated hESCs with SR-1 or TCDD to modulate AHR signaling, or DMSO vehicle control. Following 4 days of culture, hESCs treated with 1 μM SR-1 had reduced expression of CYP1A1 and CYP1B1, whereas hESCs treated with TCDD yielded significantly increased expression of CYP1A1 and CYP1B1 as compared with DMSO-normalized controls (supplemental Figure 1B). We also confirmed that there were no significant cytotoxic effects on hESCs due to the presence of SR-1 or TCDD (supplemental Figure 1C). Together, SR-1 and TCDD can effectively be used as agents to selectively regulate AHR-mediated activity in hESCs.
We next investigated differentiating hematoendothelial cells when exposed to SR-1 or TCDD. As early as day 6+3, there was a marked increase in the total percentage of CD34+CD144+ (1.71- ± 0.22-fold; P < .05) and CD34+CD31+ (1.58- ± 0.28-fold) cells that have dual hematoendothelial cell developmental potential in SR-1–treated hESCs as compared with DMSO controls34-36 (Figure 1B-C). As differentiation continued to day 6+6, there were also notable increases in the total percentage of both CD34+CD31+ (1.62- ± 0.12-fold; P < .05) hematoendothelial cells and budding CD34+CD43+ (1.36- ± 0.12-fold) hematopoietic progenitor cells6,23,37,38 when SR-1 treatment was applied. At day 6+9, there was a significant increase in development of CD34+CD45+ hematopoietic progenitor cells23,39,40 (1.40 ± 0.11; P < .05) in the SR-1–treated cells. We also observed development of more terminally differentiated hematopoietic phenotypes (CD34−CD43+ and CD34−CD45+) when hESCs were treated with TCDD. This effect was most pronounced at the day 6+9 time point, where there was a reduction in the total percentage of CD34+CD144+ (0.33 ± 0.13), CD34+CD31+ (0.49 ± 0.06; P < .05), CD34+CD43+(0.67 ± 0.09), and CD34+CD45+ (0.44 ± 0.06; P < .05) progenitor cells with an increase in the total percentage of CD34− hematopoietic cells. We observed similar trends in identical experiments using 2 independent hiPSC lines (supplemental Figure 2A-B). Taken together, these data demonstrate that AHR inhibition with SR-1 promotes early hematoendothelial cell development from human pluripotent stem cells, whereas AHR hyperactivation with TCDD accelerates differentiation toward more terminally differentiated hematopoietic lineages.
AHR inhibition leads to functional hematopoietic progenitor cells and increased expression of key hematopoietic genes
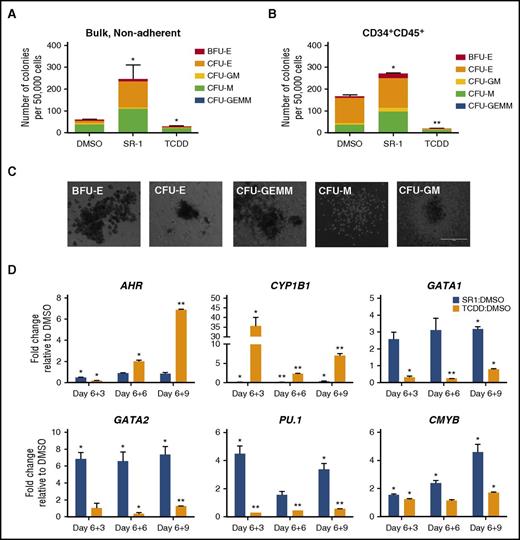
We next examined whether SR-1 supported the production of functional hematopoietic progenitor cells by standard methylcellulose-based colony-forming unit (CFU) assays. SR-1 conditioning of bulk, nonadherent hematopoietic cells derived from hESCs led to a marked increase in hematopoietic progenitor cell development compared with DMSO-treated controls (245.67 ± 64.4 colonies vs 60.3 ± 1.20 colonies, respectively; P < .05), whereas the TCDD-treated cells were significantly decreased (28.3 ± 2.67 colonies; P < .05) (Figure 2A,C). Sorted CD34+CD45+ hematopoietic progenitor cells conditioned with SR-1 yielded a similar increase in hematopoietic progenitor cell development as compared with DMSO-treated controls (270.50 ± 5.5 colonies vs 166.00 ± 6.0 colonies, respectively; P < .05) (Figure 2B), suggesting that AHR functions intrinsically at the level of hematopoietic progenitor cells to enhance hemogenic potential.
SR-1–treated hESCs demonstrate increased multilineage hematopoietic development. (A) Bulk, nonadherent hematopoietic progenitor cells and (B) sorted CD34+CD45+ cells derived from hESCs differentiated in the presence of SR-1, TCDD, or DMSO controls were harvested at day 6+5 and seeded at 50 000 cells per dish in a standard methylcellulose CFU assay. Colonies were counted for each treatment group following 2 weeks of culture and scored for the following morphological subsets: burst-forming unit-erythroid (BFU-E); CFU-E; CFU–granulocyte, macrophage (CFU-GM); CFU–granulocyte, erythroid, macrophage, megakaryocyte (CFU-GEMM); CFU-M; n = 3, error bars represent SEM of the total number of colonies per 50 000 cells seeded. *P < .05, **P < .01 as assessed with 1-way analysis of variance (ANOVA) + Tukey-Kramer multiple comparisons post hoc test. (C) Representative BFU-E, CFU-E, CFU-GEMM, CFU-M, and CFU-GM as scored in panels A and B are shown. Scale bar, 100 μm. (D) Nonadherent hematopoietic progenitor cells derived from hESCs differentiated in the presence of SR-1, TCDD, or DMSO controls were harvested at day 6+3, day 6+6, and day 6+9 time points and probed for gene expression by qRT-PCR. For each gene, cycle threshold (Ct) values were normalized to GAPDH at each time point and data are presented as relative fold change to DMSO-treated controls; n = 3, error bars represent SEM; *P < .05, **P < .01 using the Student t test.
SR-1–treated hESCs demonstrate increased multilineage hematopoietic development. (A) Bulk, nonadherent hematopoietic progenitor cells and (B) sorted CD34+CD45+ cells derived from hESCs differentiated in the presence of SR-1, TCDD, or DMSO controls were harvested at day 6+5 and seeded at 50 000 cells per dish in a standard methylcellulose CFU assay. Colonies were counted for each treatment group following 2 weeks of culture and scored for the following morphological subsets: burst-forming unit-erythroid (BFU-E); CFU-E; CFU–granulocyte, macrophage (CFU-GM); CFU–granulocyte, erythroid, macrophage, megakaryocyte (CFU-GEMM); CFU-M; n = 3, error bars represent SEM of the total number of colonies per 50 000 cells seeded. *P < .05, **P < .01 as assessed with 1-way analysis of variance (ANOVA) + Tukey-Kramer multiple comparisons post hoc test. (C) Representative BFU-E, CFU-E, CFU-GEMM, CFU-M, and CFU-GM as scored in panels A and B are shown. Scale bar, 100 μm. (D) Nonadherent hematopoietic progenitor cells derived from hESCs differentiated in the presence of SR-1, TCDD, or DMSO controls were harvested at day 6+3, day 6+6, and day 6+9 time points and probed for gene expression by qRT-PCR. For each gene, cycle threshold (Ct) values were normalized to GAPDH at each time point and data are presented as relative fold change to DMSO-treated controls; n = 3, error bars represent SEM; *P < .05, **P < .01 using the Student t test.
We further assessed key transcriptional regulators of human hematopoiesis that may be modulated by AHR expression within developing hematoendothelial cells. We again analyzed the nonadherent hematopoietic fractions of differentiating hESC-derived cells treated with DMSO, SR-1, and TCDD and performed qRT-PCR probing for AHR-related genes (AHR, CYP1B1), megakaryotic-erythropoietic genes41 (GATA1 and GATA2), a myelopoiesis regulator (PU.1),41 and a definitive hematopoiesis-specific gene (CMYB).16,42,43 We found that SR-1 treatment increased the expression of GATA1 (2.58- ± 0.40-fold) and GATA2 (6.85- ± 0.74-fold; P < .05) as early as day 6+3 (Figure 2D). The mean GATA2:GATA1 at day 6+3 was 2.67, and this positive ratio is in accord with the elevated GATA2 endogenous gene progression relative to GATA1 throughout early erythropoiesis.44,45 TCDD treatment decreased the expression of GATA1 at day 6+3 (0.31- ± 0.08-fold; P < .05) as compared with DMSO controls and induced a reduction in GATA2 later at day 6+6 (0.37- ± 0.13-fold; P < .05). There was a similar induction of PU.1 with SR-1 treatment and reciprocal expression in TCDD-treated hematopoietic cells at each time point. The increased fold change of GATA1/GATA2 and PU.1 expression supports the enhanced production of CFU-erythroid (CFU-E) and CFU-macrophage (CFU-M) in SR-1–treated hematopoietic progenitor cells (Figure 2A). Moreover, SR-1 treatment also mediated a significant increase of CMYB at all time points. To further assess whether AHR modulation influenced the hemogenic gene profile of antecedent hematoendothelial populations, we performed a similar analysis using hESC-derived CD34+CD43− sorted cells at day 6+3 of differentiation (supplemental Figure 3A). Here, we found that AHR hyperactivation with TCDD treatment reduced the relative expression of GATA1, GATA2, and PU.1, with modest increases in GATA1 and GATA2 expression with AHR inhibition with SR-1 (supplemental Figure 3B). These data suggest AHR signaling may also influence hematopoietic specification even prior to the development of early hematoendothelial cells. Collectively, these results further demonstrate that AHR inhibition leads to enhanced activation of a functional and multilineage hematopoietic transcriptional program from hESCs.
AHR-mediated differentiation of hESC-derived hematoendothelial cells is not due to increased proliferation of CD34+CD45+ cells
We next evaluated whether SR-1–mediated enhancement of CD34+CD45+ cells was due to increased cellular proliferation, as is the case in CD34+CD45+ from human UCB. The absolute number of UCB-derived CD34+CD45+ cells treated with DMSO, SR-1, and TCDD expanded at a significantly greater rate than CD34+CD45+ cells sorted from day 6+5 hESCs (supplemental Figure 4A). At day 15 of culture, there was a significant increase in the absolute number of cells in the UCB SR-1 treatment (14.2 × 106 ± 1.0 × 106; P < .05) relative to both DMSO (9.00 × 106 ± 0.71 × 106) and TCDD (7.14 × 106 ± 0.46 × 106) treatment, in addition to the total number of CD34+CD45+ cells (DMSO, 1.69 × 106 ± 0.13 × 106; SR-1, 5.62 × 106 ± 0.40 × 106 [P < .05]; TCDD, 0.15 × 106 ± 0.01 × 106), which is consistent with previous reports.46,47 However, we did not find a similar expansion of day 6+5 hESC-derived CD34+CD45+ at any time point. TCDD treatment of both UCB and hESC-derived CD34+CD45+ accelerated the differentiation to terminally differentiated CD34− hematopoietic cells as compared with DMSO, whereas SR-1 slowed this progression and retained hematopoietic cells in a progenitor state (supplemental Figure 4B). Together, these data suggest that hESC-derived hematoendothelial progenitor cells do not proliferate in response to SR-1 as do UCB CD34+ cells, but rather are enhanced through differentiation mechanisms from early hematoendothelial progenitors.
AHR knockout in hESCs promotes early hematopoietic differentiation
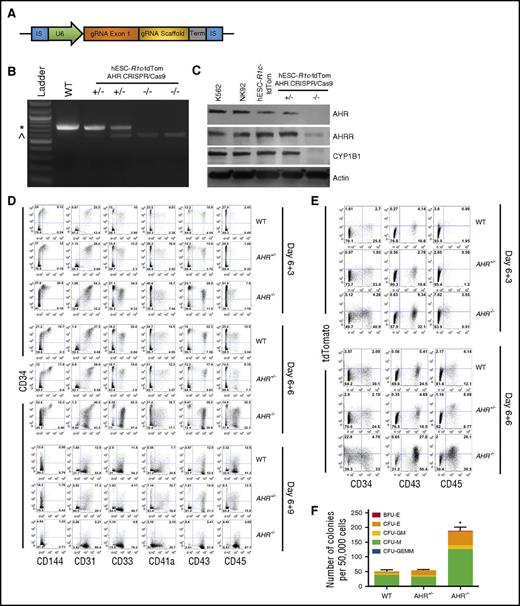
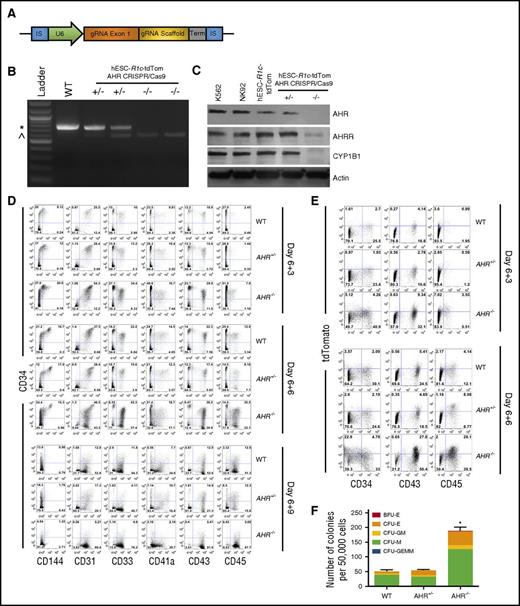
CRIPSR/Cas9-mediated gene deletion provides a more targeted approach to define the role of AHR during early human hematoendothelial and hematopoietic progenitor cell production. Here, we used CRIPSR/Cas9 to develop stable and clonally derived hESC cell lines with a targeted AHR deletion. Specifically, we used hESCs previously modified with a RUNX1c-tdTomato reporting cassette generated in our laboratory that demonstrates faithful measurement of early hematoendothelial cells.23 As previously described, these cells allowed us to observe EHT and isolate early human hematopoietic cells as they emerge from adherent endothelial cells. These cells now allow us to directly evaluate the effect of AHR gene modification on the induction of EHT.16,23 We clonally expanded hESCs transfected with a gRNA target complementary to AHR exon 1 and probed for modification using primers flanking the exon 1 sequence (Figure 3A). We identified clones that yielded a 718-bp amplicon (wild-type [WT]), a 718-bp amplicon with an additional 571-bp amplicon indicative of partial exon 1 deletion (AHR+/−), and only the 571-bp amplicon (AHR−/−) (Figure 3B). We confirmed functional loss of AHR protein with significant attenuation of the AHR-downstream genes AHR repressor (AHRR) and CYP1B1 in AHR−/−-hESC-RUNX1c-tdTomato cells, as compared with K562+ and NK92+ controls, and WT hESC-RUNX1c-tdTomato cells (Figure 3C). We additionally validated the on-target specificity of the gRNA by probing the AHR amplicons generated from the genomic DNA of each clone with Surveyor endonuclease as well as with direct sequencing (data not shown). These data confirmed that we successfully generated heterozygous and homozygous deletions of AHR within hESCs.
CRISPR/Cas9-engineered hESCs with AHR deletion demonstrate increased early hematoendothelial cell development. (A) gRNA cassette design targeting AHR. gRNA exon 1 indicates 22-nt gRNA specific to AHR exon 1. (B) Gel electrophoresis of PCR products produced from clonally derived hESC-RUNx1c-tdTomato cells nucleofected with AHR gRNA cassette. Genomic DNA was harvested and primers flanking the AHR exon 1 locus were used to generate a PCR product with predicted full-length of 718 bp. WT indicates negatively selected nucleofected hESC-RUNX1c-tdTomato hESCs; +/− indicates individual clones with AHR heterozygous deletion (AHR+/−); −/− indicates individual clones with AHR homozygous deletion (AHR−/−). (C) Immunoblot of protein lysate harvested from K562 cells (positive control), NK92 NK cells (positive control), WT hESC-RUNX1c-tdTomato (hESC-R1c-tdTom), heterozygous AHR-deleted hESC-RUNX1c-tdTomato (+/−), and homozygous AHR deleted hESC-RUNX1c-tdTomato (−/−). (D) Representative flow cytometry plots at day 6+3, day 6+6, and day 6+9 from 1 differentiation of WT hESC-RUNX1c-tdTomato (WT), heterozygous AHR hESC-RUNX1c-tdTomato deletion (AHR+/−), and homozygous AHR hESC-RUNX1c-tdTomato deletion (AHR−/−). Both adherent and nonadherent cell fractions are harvested at day 6+3, day 6+6, and day 6+9 and assessed for endothelial (CD31, CD144), and hematopoietic (CD33, CD41a, CD43, CD45) phenotype. (E) Representative flow cytometry plots at day 6+3 and day 6+6 from 1 differentiation assessing for RUNX1c expression based on tdTomato fluorescent reporter protein. (F) Nonadherent hematopoietic progenitor cells derived from WT hESC-RUNX1c-tdTomato, AHR+/− hESC-RUNX1c-tdTomato, or AHR−/− hESC-RUNX1c-tdTomato were harvested at day 6+5 and seeded at 50 000 cells per dish in a standard methylcellulose CFU assay (CFU). Colonies were counted for each treatment group following 2 weeks of culture and scored for the following morphological subsets, as previously described; n = 3, error bars represent SEM of the total number of colonies per 50 000 cells seeded. *P < .05 as assessed with 1-way ANOVA + Tukey-Kramer multiple comparisons post hoc test. IS, insertion sequence; Term, termination sequence. *718-bp amplicon; ^571-bp amplicon.
CRISPR/Cas9-engineered hESCs with AHR deletion demonstrate increased early hematoendothelial cell development. (A) gRNA cassette design targeting AHR. gRNA exon 1 indicates 22-nt gRNA specific to AHR exon 1. (B) Gel electrophoresis of PCR products produced from clonally derived hESC-RUNx1c-tdTomato cells nucleofected with AHR gRNA cassette. Genomic DNA was harvested and primers flanking the AHR exon 1 locus were used to generate a PCR product with predicted full-length of 718 bp. WT indicates negatively selected nucleofected hESC-RUNX1c-tdTomato hESCs; +/− indicates individual clones with AHR heterozygous deletion (AHR+/−); −/− indicates individual clones with AHR homozygous deletion (AHR−/−). (C) Immunoblot of protein lysate harvested from K562 cells (positive control), NK92 NK cells (positive control), WT hESC-RUNX1c-tdTomato (hESC-R1c-tdTom), heterozygous AHR-deleted hESC-RUNX1c-tdTomato (+/−), and homozygous AHR deleted hESC-RUNX1c-tdTomato (−/−). (D) Representative flow cytometry plots at day 6+3, day 6+6, and day 6+9 from 1 differentiation of WT hESC-RUNX1c-tdTomato (WT), heterozygous AHR hESC-RUNX1c-tdTomato deletion (AHR+/−), and homozygous AHR hESC-RUNX1c-tdTomato deletion (AHR−/−). Both adherent and nonadherent cell fractions are harvested at day 6+3, day 6+6, and day 6+9 and assessed for endothelial (CD31, CD144), and hematopoietic (CD33, CD41a, CD43, CD45) phenotype. (E) Representative flow cytometry plots at day 6+3 and day 6+6 from 1 differentiation assessing for RUNX1c expression based on tdTomato fluorescent reporter protein. (F) Nonadherent hematopoietic progenitor cells derived from WT hESC-RUNX1c-tdTomato, AHR+/− hESC-RUNX1c-tdTomato, or AHR−/− hESC-RUNX1c-tdTomato were harvested at day 6+5 and seeded at 50 000 cells per dish in a standard methylcellulose CFU assay (CFU). Colonies were counted for each treatment group following 2 weeks of culture and scored for the following morphological subsets, as previously described; n = 3, error bars represent SEM of the total number of colonies per 50 000 cells seeded. *P < .05 as assessed with 1-way ANOVA + Tukey-Kramer multiple comparisons post hoc test. IS, insertion sequence; Term, termination sequence. *718-bp amplicon; ^571-bp amplicon.
We next differentiated WT-, AHR+/−-, and AHR−/−-RUNX1c-tdTomato hESCs as in previous studies. At day 6+3, there was approximately a twofold increase in development of hematoendothelial cells (CD34+CD31+ and CD34+CD144+) as compared with WT and AHR+/− hESCs at day 6+3 (Figure 3D; quantified in supplemental Figure 5A). We also found that AHR−/−-RUNX1c-tdTomato hESCs produced more than a twofold increase in CD34+CD43+ and CD34+CD45+ hematopoietic progenitor cells at both day 6+3 and day 6+6 time points. Importantly, the total percentage of CD34+ was not compromised as hematopoietic progenitor cells further differentiated into mature hematopoietic cells (CD34−CD33+, CD34−CD41a+, CD34−CD43+, CD34−CD45+). By day 6+9, a majority of the AHR−/− hESC-derived cells continued to differentiate into mature hematopoietic lineages at a greater rate than WT and AHR+/− hESCs, as indicated by an increased total percentage of CD34−CD45+ cells.
Using the RUNX1c-tdTomato reporter, we also demonstrated an increased commitment toward RUNX1c+ cell development in AHR−/−-RUNX1c-tdTomato hESCs as compared with WT- and AHR+/−-RUNX1c-tdTomato hESCs. Specifically, there was a fivefold expansion in the total percentage of tdTomato+ hematopoietic progenitor cells at both day 6+3 and day 6+6 in AHR−/−-RUNX1c-tdTomato hESCs compared with WT- and AHR+/−-RUNX1c-tdTomato hESCs (Figure 3E; quantified in supplemental Figure 5B). We also further confirmed increased development of functional hematopoietic progenitor cells derived from AHR−/−-RUNX1c-tdTomato hESCs compared with the controls using hematopoietic CFU assays. There was a significant increase in the total number of colonies formed in the AHR−/− hESCs (188.67 ± 11.29 colonies; P < .05) as compared with AHR+/− hESCs (54.0 ± 2.08 colonies) and WT hESCs (50.33 ± 4.91 colonies) (Figure 3F). Collectively, these data demonstrate that genetic deletion of AHR in hESCs mediates a significant increase in functional hematoendothelial differentiation.
AHR inhibition enhances cNK cell differentiation from hESCs whereas AHR hyperactivation supports ILC3 cell phenotype
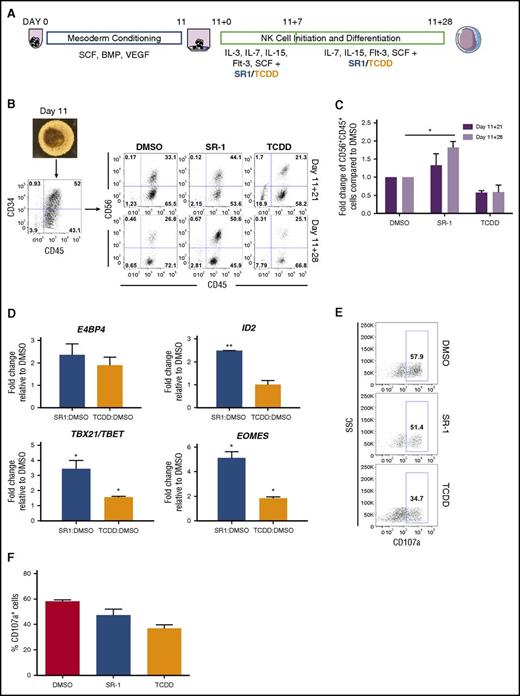
Recent studies also demonstrate an important role of AHR: to mediate development and function of both innate and adaptive immune cells.48,49 Because AHR attenuation supports the differentiation of CD34+CD45+ hematopoietic progenitor cells (Figures 1B and 3D), we assessed whether NK-cell differentiation could also be enhanced from hESCs using defined conditions and a small-molecule approach. Here, we used our previously described system for NK-cell development from hESCs as a model for lymphopoiesis10,11,25 (Figure 4A). By day 11, spin EBs produced a high percentage of CD34+CD45+ hematopoietic progenitor cells (range, 38.5%-65.0% for n = 3 separate studies) (Figure 4B). At days 11+21 (11 days in hematopoietic differentiation conditions, then 21 days in NK-cell differentiation conditions) and day 11+28, SR-1–treated hESC-derived hematopoietic cells demonstrated increased development of NK cells compared with DMSO-treated controls, whereas TCDD-treated hESC-derived hematopoietic cells had fewer phenotypic NK cells (Figure 4B-C). In addition to surface antigen acquisition, we also assessed lymphoid-specific gene expression in the hematopoietic cells produced in each treatment group. Compared with the DMSO-treated control group, SR-1–treated hESC-derived hematopoietic cells expressed a significantly higher amount of ID2 (2.49- ± 0.003-fold; P < .01), TBX21/TBET (3.44- ± 0.55-fold; P < .05), and EOMES (5.12- ± 0.52-fold; P < .05), transcriptional factors that mediate increased NK-cell lineage commitment (Figure 4D). Although we also observed a significant increase in TBX21/TBET (1.56- ± 0.07-fold; P < .05) and EOMES (1.84- ± 0.11-fold; P < .05) in the TCDD-treated hESC-derived hematopoietic cells, the fold induction was significantly lower than those of the SR-1–treated group. We further assessed the function of differentiated NK cells by assessing CD107a degranulation when stimulated with K562 target cells. SR-1–treated hESC-derived hematopoietic cells were comparable to DMSO-treated controls in CD107a expression (58.1% ± 0.67% vs 47.2% ± 2.76%), whereas TCDD-treated hESC-derived hematopoietic cells expressed less CD107a (36.8% ± 2.1%) (Figure 4E-F). Collectively, these data support SR-1 treatment of differentiating hESCs to enhance the production of functional NK cells.
hESCs differentiated in the presence of SR-1 promote the development of functional NK cells. (A) Schema of hESC differentiation into lymphoid cells as spin EBs. hESCs are made into spin EBs at day 0 and cultured in stage 1 conditions with defined cytokines to promote mesoderm development for 11 days. At day 11, spin EBs are transferred onto OP9-DL1 in the presence of NKDM to promote lymphoid differentiation. Cells are treated beginning at day 11+0 with either 1 μM SR-1, 10 nM TCDD, or DMSO vehicle control with media exchanges and/or harvesting performed every week for up to 4 weeks. (B) At day 11, differentiated spin EBs (original magnification ×40) are phenotyped for CD34+CD45+ expression and transferred to OP9-DL1 stroma in NKDM. Nonadherent hematopoietic cells cultured either in the presence of DMSO, SR-1, or TCDD were assessed for developing NK-cell immunophenotype based on CD56+CD45+ expression at days 11+21, and 11+28; representative flow cytometry plots from 1 differentiation are shown. (C) Quantification of fold change in total percentage of CD56+CD45+ cells at both day 11+21 and day 11+28 when treated with DMSO, SR-1, or TCDD. SR-1 and TCDD treatments for each differentiation are normalized to DMSO controls; n = 3 independent differentiation experiments, error bars represent SEM. *P < .05 as assessed with 2-way ANOVA + Tukey-Kramer multiple comparisons post hoc test. (D) Nonadherent hematopoietic progenitor cells derived from hESCs differentiated in the presence of SR-1, TCDD, or DMSO controls were harvested at day 11+28 and probed for gene expression by qRT-PCR. For each gene, Ct values were normalized to GAPDH at each time point and data are presented as relative fold change to DMSO-treated controls; n = 3, error bars represent SEM. *P < .05; #P < .01 using the Student t test. (E) Nonadherent hematopoietic progenitor cells derived from hESCs differentiated in the presence of SR-1, TCDD, or DMSO controls were harvested at day 11+28 and assessed for CD107a expression following 4 hours of coculture with K562 target cells at a 2:1 effector:target ratio. Representative flow cytometry plots are shown from 1 experiment. (F) Quantification of percentage of CD107a+ cells when treated with DMSO, SR-1, or TCDD at day 11+28; n = 2-3 replicates. SSC, side scatter.
hESCs differentiated in the presence of SR-1 promote the development of functional NK cells. (A) Schema of hESC differentiation into lymphoid cells as spin EBs. hESCs are made into spin EBs at day 0 and cultured in stage 1 conditions with defined cytokines to promote mesoderm development for 11 days. At day 11, spin EBs are transferred onto OP9-DL1 in the presence of NKDM to promote lymphoid differentiation. Cells are treated beginning at day 11+0 with either 1 μM SR-1, 10 nM TCDD, or DMSO vehicle control with media exchanges and/or harvesting performed every week for up to 4 weeks. (B) At day 11, differentiated spin EBs (original magnification ×40) are phenotyped for CD34+CD45+ expression and transferred to OP9-DL1 stroma in NKDM. Nonadherent hematopoietic cells cultured either in the presence of DMSO, SR-1, or TCDD were assessed for developing NK-cell immunophenotype based on CD56+CD45+ expression at days 11+21, and 11+28; representative flow cytometry plots from 1 differentiation are shown. (C) Quantification of fold change in total percentage of CD56+CD45+ cells at both day 11+21 and day 11+28 when treated with DMSO, SR-1, or TCDD. SR-1 and TCDD treatments for each differentiation are normalized to DMSO controls; n = 3 independent differentiation experiments, error bars represent SEM. *P < .05 as assessed with 2-way ANOVA + Tukey-Kramer multiple comparisons post hoc test. (D) Nonadherent hematopoietic progenitor cells derived from hESCs differentiated in the presence of SR-1, TCDD, or DMSO controls were harvested at day 11+28 and probed for gene expression by qRT-PCR. For each gene, Ct values were normalized to GAPDH at each time point and data are presented as relative fold change to DMSO-treated controls; n = 3, error bars represent SEM. *P < .05; #P < .01 using the Student t test. (E) Nonadherent hematopoietic progenitor cells derived from hESCs differentiated in the presence of SR-1, TCDD, or DMSO controls were harvested at day 11+28 and assessed for CD107a expression following 4 hours of coculture with K562 target cells at a 2:1 effector:target ratio. Representative flow cytometry plots are shown from 1 experiment. (F) Quantification of percentage of CD107a+ cells when treated with DMSO, SR-1, or TCDD at day 11+28; n = 2-3 replicates. SSC, side scatter.
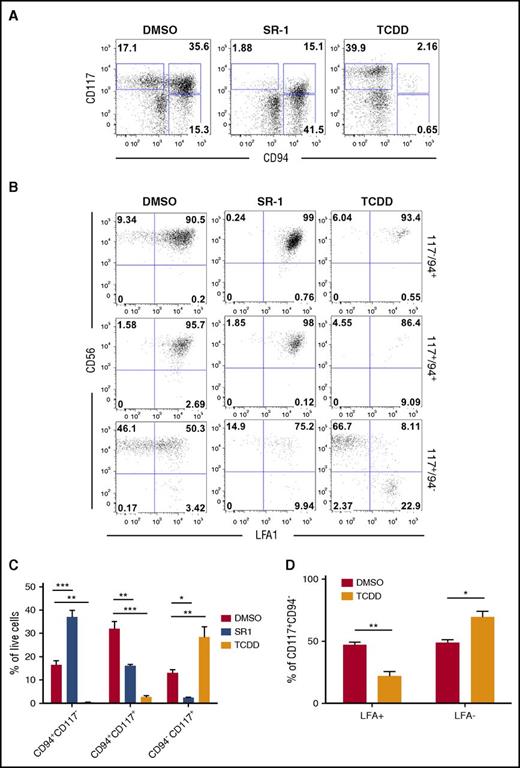
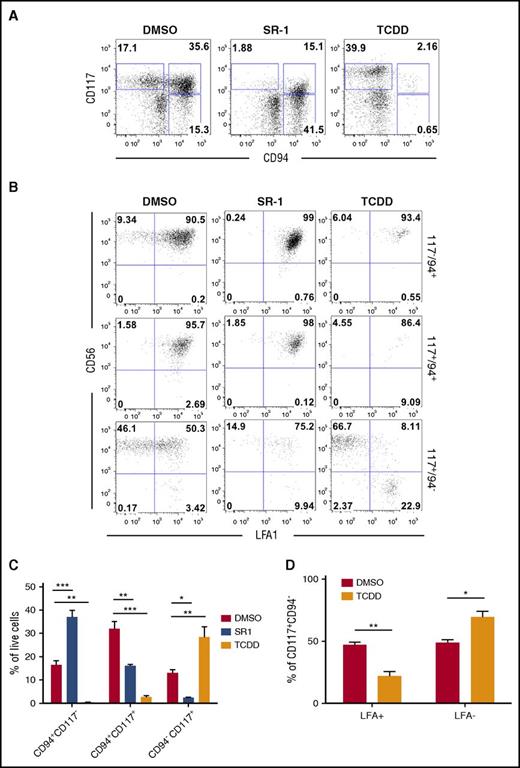
We further defined the identity of developing lymphoid phenotypes regulated by AHR activity by evaluating natural killer progenitor (NKP) cells, cNK cells, and developmentally related ILC3 cells.50-52 At day 11+28, hESC-derived hematopoietic cells treated with DMSO control produced cNK (CD94+CD117−CD56+LFA1+) and ILC3 (CD94−CD117+CD56+LFA1−) cells, but with a majority of the differentiated cells restricted to the NKP (CD94+CD117+) gate (Figure 5A-B; gating schema supplemental Figure 6A). Treatment with SR-1 significantly shifted hESC-derived hematopoietic cells away from NKPs (16.13% ± 0.58% vs 32.0% ± 2.98%; P < .01) and toward cNK cells (37.0% ± 2.92% vs 16.5% ± 1.77%; P < .001) compared with DMSO, with a significant reduction in the CD94−CD117+ population (Figure 5C). Treatment with TCDD also significantly shifted hESC-derived hematopoietic cells away from NKPs and led to a reciprocal increase in CD94−CD117+ cells (28.5% ± 4.42% vs 13.1% ± 1.34%; P < .01). When CD94−CD117+ cells were gated to distinguish the presence of ILC3s, a significantly larger percentage of TCDD-treated hESC-derived hematopoietic cells were absent for LFA1, as compared with DMSO-treated controls (69.4% ± 4.57% vs 48.8% ± 4.81%; P < .05) (Figure 5D). We have previously shown that LFA1 expression is a unique and distinguishing marker between ILC3 (LFA1−) and cNK (LFA1+).52 Similar effects on cNK/ILC3 differentiation were observed when using CD34+ cells derived from human UCB (supplemental Figure 6B). We next sorted populations of cNK, NKP, and ILC3 and performed qRT-PCR to assess for both NK- and ILC3-specific gene expression (supplemental Figure 6C). As expected, hESC-derived phenotypic ILC3 cells had a classical ILC3 gene signature, specifically RORc, IL1-R1, and IL-22. Additionally, ILC3-sorted populations were virtually deficient for GATA3, a critical transcriptional regulator of group 2 ILCs,53 and were decreased for TBX21/TBET expression (supplemental Figure 6D). These data further support the hypothesis that AHR inhibition promotes the differentiation of NK progenitor cells into mature cNK cells. Furthermore, for the first time, we demonstrate AHR hyperactivation promotes development of an ILC3 phenotype from hESCs.
hESCs differentiated in the presence of SR-1 skews development toward cNK cells whereas TCDD supports the development of an ILC phenotype. (A) Representative flow cytometry profile of nonadherent hematopoietic cells differentiated from hESCs in the presence of DMSO, SR-1, or TCDD at day 11+28. (B) cNK, NKP, and NKP/ILC subpopulations from day 11+28 DMSO-, SR-1–, and TCDD-differentiated hESCs assessed for CD56 and LFA (CD11a/CD18) surface antigen expression. Representative flow cytometry plots are shown; n = 3. (C) Total percentage of cNK, NKP, and NKP/ILCs present in the nonadherent fraction of differentiating hESCs in the presence of DMSO, SR-1, or TCDD at day 11+28; n = 3, error bars represent SEM. *P < .05, **P < .01, ***P < .001 as compared with DMSO-treated controls and assessed by 2-way ANOVA + Tukey-Kramer multiple comparisons post hoc test. (D) CD94−CD117+ subpopulations were further quantified for expression of LFA+ (NKP) and LFA− (ILC) by flow cytometry; n = 3, error bars represent SEM. *P < .05, **P < .01 as compared with DMSO-treated controls and assessed by 2-way ANOVA + the Tukey-Kramer multiple comparisons post hoc test.
hESCs differentiated in the presence of SR-1 skews development toward cNK cells whereas TCDD supports the development of an ILC phenotype. (A) Representative flow cytometry profile of nonadherent hematopoietic cells differentiated from hESCs in the presence of DMSO, SR-1, or TCDD at day 11+28. (B) cNK, NKP, and NKP/ILC subpopulations from day 11+28 DMSO-, SR-1–, and TCDD-differentiated hESCs assessed for CD56 and LFA (CD11a/CD18) surface antigen expression. Representative flow cytometry plots are shown; n = 3. (C) Total percentage of cNK, NKP, and NKP/ILCs present in the nonadherent fraction of differentiating hESCs in the presence of DMSO, SR-1, or TCDD at day 11+28; n = 3, error bars represent SEM. *P < .05, **P < .01, ***P < .001 as compared with DMSO-treated controls and assessed by 2-way ANOVA + Tukey-Kramer multiple comparisons post hoc test. (D) CD94−CD117+ subpopulations were further quantified for expression of LFA+ (NKP) and LFA− (ILC) by flow cytometry; n = 3, error bars represent SEM. *P < .05, **P < .01 as compared with DMSO-treated controls and assessed by 2-way ANOVA + the Tukey-Kramer multiple comparisons post hoc test.
Discussion
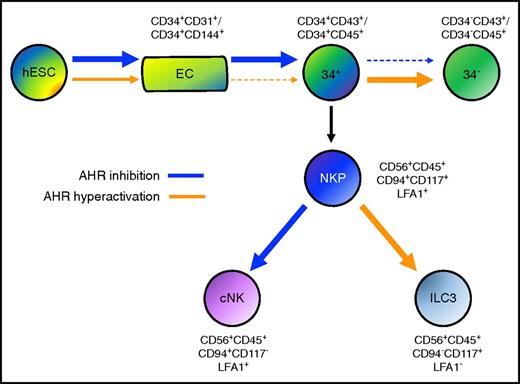
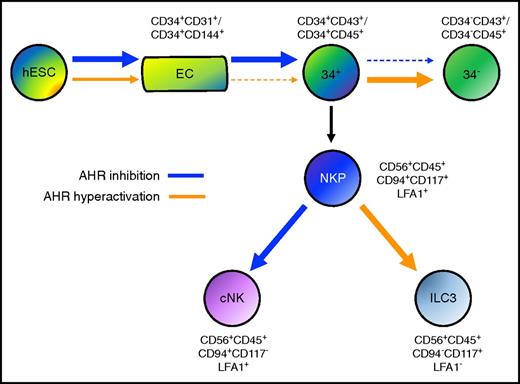
Human pluripotent stem cells provide an important starting point to better define key molecular and genetic drivers of human hematoendothelial development. Here, we established that AHR antagonism using the chemical inhibitor SR-1, as well as AHR gene deletion using the CRIPSR/Cas9 system, enhances human EHT and hematopoietic progenitor cell development. In corresponding fashion, AHR hyperactivation using TCDD suppresses development of hematopoietic progenitor cells with multilineage potential and accelerates their differentiation into more matured hematopoietic lineages (Figure 6).
Model of AHR activity in human developmental hematopoiesis. AHR inhibition mediated by SR-1 (blue arrow) enhances the differentiation of both endothelial cells (ECs) and CD34+ hematopoietic progenitor cells. AHR hyperactivation mediated by TCDD (orange arrow) reciprocally acts to attenuate both ECs and CD34+ hematopoietic progenitor cells. Once CD34+ has been differentiated, AHR inhibition deters further differentiation into CD34− terminally matured hematopoietic cells, whereas AHR hyperactivation supports this process. Upon production of NKP cells, AHR inhibition promotes cNK cell differentiation (cNK), whereas AHR hyperactivation promotes ILC3 differentiation.
Model of AHR activity in human developmental hematopoiesis. AHR inhibition mediated by SR-1 (blue arrow) enhances the differentiation of both endothelial cells (ECs) and CD34+ hematopoietic progenitor cells. AHR hyperactivation mediated by TCDD (orange arrow) reciprocally acts to attenuate both ECs and CD34+ hematopoietic progenitor cells. Once CD34+ has been differentiated, AHR inhibition deters further differentiation into CD34− terminally matured hematopoietic cells, whereas AHR hyperactivation supports this process. Upon production of NKP cells, AHR inhibition promotes cNK cell differentiation (cNK), whereas AHR hyperactivation promotes ILC3 differentiation.
To our knowledge, no other study has reported on the ability of AHR inhibition to promote early human hematoendothelial differentiation. Gori et al assessed the effect of AHR inhibition using short-term SR-1 treatment in a nonhuman primate iPSC model of hematopoiesis.54 Although these studies showed an increase in phenotypic CD34+CD45+ cells, there were no differences in the kinetics or quantity of CD34+ or CD34+CD31+ cells. SR-1–treated nonhuman primate iPSCs also did not enhance the total number of CFUs, unlike our findings using hESCs. These differences may be due to differences in culture conditions and/or possible species-specific differences. Indeed, AHR ligand selectivity and AHR interaction with coactivator motifs all substantially differ between nonhuman and primary human cells.55-57 Furthermore, our findings suggest that AHR inhibition plays a critical role in the hemogenecity of differentiating hematoendothelial cells as opposed to facilitating cell expansion. Unlike UCB, hESC-derived CD34+CD45+ cells did not robustly expand, but alternatively significantly enhanced hematopoietic progenitor cell development, as assessed via CFU assay. These findings provide complementary data to our model in that AHR acts as an integral component to facilitate hematopoietic differentiation and progenitor cell maturation throughout developmental hematopoiesis.
Interestingly, we also demonstrate that AHR gene deletion enhances development of early hematopoietic progenitor cells as they differentiate into definitive hematopoietic lineages. We, and others, have reported that the RUNX1c isoform is correlated with emerging definitive HSPCs from aorta-gonad-mesonephros-region endothelial cells.23,42 Using our previously developed RUNX1c-tdTomato reporter system to model EHT, we found that hESCs harboring AHR gene deletion enhanced the differentiation of RUNX1c+ hematopoietic cells. We also observed induction of a multilineage transcriptional program, including typical genes expressed during definitive hematopoiesis. One hypothesis for this effect is that AHR may function as a modulator of β-catenin/Wnt signaling. Exogenous activation of Wnt through GSK3 inhibition has been recently shown to support a definitive hematopoietic phenotype from human pluripotent stem cells.58 Interestingly, AHR and Wnt signaling have known associations both in normal embryological and disease pathogenesis. β-catenin gene expression (CTNNB1) is known to be overexpressed in Ahr−/− mice, causing the development of intestinal tumors.59,60 Another study also demonstrated that Ahr−/− mice had increased expression of genes regulated by β-catenin/Wnt signaling, specifically within hematopoietic stem cells (HSCs).61 These Ahr−/− mice demonstrated splenomegaly, anemia, leukocytosis, and HSC accumulation outside of the bone marrow. Together, these studies all implicate AHR as a potent regulator of definitive hematopoiesis.
In addition to increased development of hematoendothelial cells, we also found that differentiation of lymphoid cells (NK cells) was increased by treatment of hESCs with SR-1. These findings are complementary to prior studies that observed that SR-1 treatment not only enhanced several transcription factors that are indispensable for NK-cell differentiation such as ID2, GATA3, and EOMES, but also promoted an increased absolute number of NK cells derived from mobilized peripheral blood CD34+ hematopoietic progenitor cells.47 Our hESC-derived NK cells in the presence of SR-1 were functional, in that they demonstrated typical degranulation (CD107a) compared with controls when stimulated with K562 targets. We further emphasize the role of AHR in hematolymphoid development by demonstrating that AHR antagonism accelerates differentiation of NK progenitor cells into cNK phenotypes. This study illustrates that SR-1 can be added into currently defined differentiation protocols to enhance the efficiency and homogeneity of hESC-derived NK cells suitable to human clinical trials.
Finally, to our knowledge, this is the first report demonstrating that ILC3s can be derived from human pluripotent stem cells. hESC-derived ILC3s, like those located in secondary lymphoid tissue and peripheral blood, require AHR to drive their development.52,62,63 Several studies have highlighted the critical immunomodulatory role ILC3s play in the gut mucosa, specifically in the production of IL-22, which is required for intestinal homeostasis.64 It remains unclear whether they can be harnessed for clinical application, such as the attenuation of acute graft-vs-host disease post-HSC transplantation.65 hESCs, particularly in conjunction with a CRISPR/Cas9 gene-editing system as we present, can be used as a powerful platform to better understand the development of a range of human ILCs, as well as to better analyze their effector phenotypes and therapeutic potential.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Anna Kim for technical assistance.
This work was supported in part by the following National Institutes of Health grants: National Cancer Institute R01CA203348 (D.S.K.), National Institute of Diabetes and Digestive and Kidney Diseases F30DK107071 (M.G.A.), National Institute of Allergy and Immunology R01AI100879 (M.R.V.), and National Institute of General Medicine Sciences T32GM113846 (M.G.A. and D.S.K.) and T32GM008244 (M.G.A.). Other support was received from the Regenerative Medicine Minnesota program (D.S.K.).
Authorship
Contribution: M.G.A. designed the experimental plan, performed the experiments, analyzed the data, and wrote the manuscript; B.R.W. designed the CRISPR gRNA and performed hESC nucleofection; P.N.R., R.H.B., C.D.R., L.B., S.S., A.M.Y., and D.M.T. performed the experiments and provided technical assistance; M.R.V. edited the manuscript; and D.S.K. designed the experimental plan and wrote and edited the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Dan S. Kaufman, Division of Regenerative Medicine, Department of Medicine, University of California San Diego, 9500 Gilman Dr, MC 0695, La Jolla, CA 92093-0695; e-mail: dskaufman@ucsd.edu.
References
Author notes
M.R.V. and D.S.K. contributed equally.