Abstract
Since their discovery, immunoreceptor tyrosine-based inhibition motif (ITIM)-containing receptors have been shown to inhibit signaling from immunoreceptor tyrosine-based activation motif (ITAM)-containing receptors in almost all hematopoietic cells, including platelets. However, a growing body of evidence has emerged demonstrating that this is an oversimplification, and that ITIM-containing receptors are versatile regulators of platelet signal transduction, with functions beyond inhibiting ITAM-mediated platelet activation. PECAM-1 was the first ITIM-containing receptor identified in platelets and appeared to conform to the established model of ITIM-mediated attenuation of ITAM-driven activation. PECAM-1 was therefore widely accepted as a major negative regulator of platelet activation and thrombosis for many years, but more recent findings suggest a more complex role for this receptor, including the facilitation of αIIbβ3-mediated platelet functions. Since the identification of PECAM-1, several other ITIM-containing platelet receptors have been discovered. These include G6b-B, a critical regulator of platelet reactivity and production, and the noncanonical ITIM-containing receptor TREM-like transcript-1, which is localized to α-granules in resting platelets, binds fibrinogen, and acts as a positive regulator of platelet activation. Despite structural similarities and shared binding partners, including the Src homology 2 domain-containing protein-tyrosine phosphatases Shp1 and Shp2, knockout and transgenic mouse models have revealed distinct phenotypes and nonredundant functions for each ITIM-containing receptor in the context of platelet homeostasis. These roles are likely influenced by receptor density, compartmentalization, and as-yet unknown binding partners. In this review, we discuss the diverse repertoire of ITIM-containing receptors in platelets, highlighting intriguing new functions, controversies, and future areas of investigation.
Introduction
Platelets are small, anucleate fragments derived from megakaryocytes that are vital for maintaining hemostasis. A growing body of evidence has established that platelets also contribute to other pathophysiological processes, including atherogenesis,1 inflammation,2 wound repair,3 angiogenesis,4 blood-lymphatic vessel separation,5 and cancer metastasis.6 A common underlying theme is that platelets must transition from a “resting” to an “activated” state to contribute to each of these processes. Thus, understanding how platelets undergo this transition has broad implications for a number of diseases.
Taking thrombosis and hemostasis as a paradigm of platelet activation, transition from the resting state relies on key receptors coming into contact with their cognate ligands in the vessel wall and transmitting activation signals within platelets. Once adhered, thrombus formation is initiated and shaped accordingly to minimize blood loss while maintaining blood flow. Tyrosine kinase-linked receptors, including the von Willebrand factor receptor complex glycoprotein Ib-IX-V (GPIb-IX-V) and the integrins αIIbβ3 and α2β1 play critical roles in the initial tethering, adhesion, and activation of platelets at sites of injury. More robust activation is provided by the immunoreceptor tyrosine-based activation motif (ITAM)-containing collagen receptor complex GPVI-Fc receptor (FcR) γ-chain,7 collagen-mediated clustering of which leads to phosphorylation of tyrosine residues within the FcR γ-chain ITAM by Src family kinases (SFKs). The Src homology 2 (SH2) domain–containing protein-tyrosine kinase Syk can then be recruited and propagate the signal through tyrosine phosphorylation and a rise in intracellular Ca2+. The cellular consequences of these series of biochemical events is the release of secondary mediators, including adenosine diphosphate (ADP) and thromboxane A2, integrin activation, and procoagulant activity. Secondary signals provided by the G protein-coupled receptors for ADP (P2Y1 and P2Y12), thromboxane A2 (TP) and thrombin (PAR-1 and PAR-4), synergize with initiating signals from tyrosine kinase-linked receptors to maximally activate platelets and coordinate the platelet response with the coagulation system.
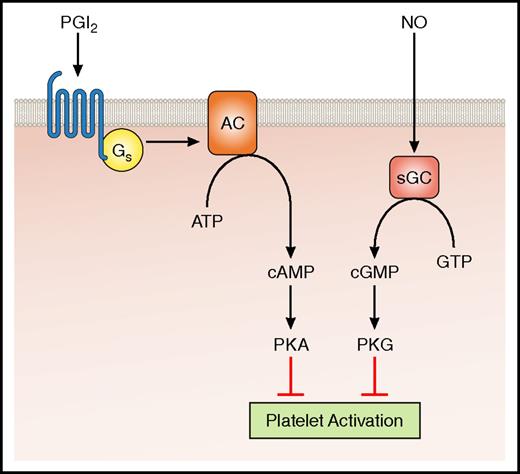
Equally important, but less well understood, are the mechanisms that prevent unwarranted platelet activation and limit thrombus size. Under normal physiological conditions, platelets are maintained in a resting state by transient inhibitory signals that prevent unnecessary activation while at the same time allowing platelets to fully respond to vascular injury when required. Two of the most powerful platelet inhibitors are prostacyclin (PGI2) and nitric oxide (NO), which are released by healthy endothelia. They act by elevating intracellular cyclic adenosine monophosphate and cyclic guanosine monophosphate levels in the platelet via adenylate cyclase and soluble guanylate cyclase, respectively. These cyclic nucleotides in turn activate protein kinase A and protein kinase G, respectively, which phosphorylate key targets and inhibit platelet activation (Figure 1).8
Inhibition of platelet activation by cyclic nucleotide generation. PGI2 and NO activation of cyclic nucleotide generation provide generalized inhibition of platelet activation. AC, adenylate cyclase; ATP, adenosine triphosphate; cAMP, cyclic adenosine monophosphate; cGMP, cyclic guanosine monophosphate; Gs, guanine nucleotide-binding protein stimulatory; GTP, guanosine triphosphate; PKA, protein kinase A; PKG, protein kinase G; sGC, soluble guanlyate cyclase. Professional illustration by Patrick Lane, ScEYEnce Studios.
Inhibition of platelet activation by cyclic nucleotide generation. PGI2 and NO activation of cyclic nucleotide generation provide generalized inhibition of platelet activation. AC, adenylate cyclase; ATP, adenosine triphosphate; cAMP, cyclic adenosine monophosphate; cGMP, cyclic guanosine monophosphate; Gs, guanine nucleotide-binding protein stimulatory; GTP, guanosine triphosphate; PKA, protein kinase A; PKG, protein kinase G; sGC, soluble guanlyate cyclase. Professional illustration by Patrick Lane, ScEYEnce Studios.
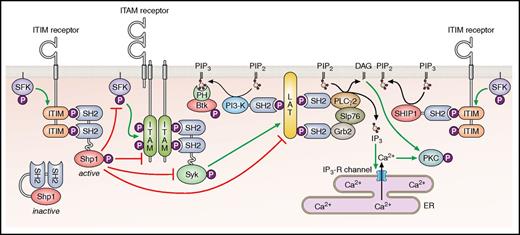
Immunoreceptor tyrosine-based inhibition motif (ITIM)-containing receptors provide a more specialized, cell-intrinsic inhibition compared with PGI2 and NO by primarily targeting ITAM-containing receptors (Figure 2). The prototype of this family of receptors is the immune receptor FcγRIIB, which inhibits signaling from the ITAM-containing B-cell receptor complex after ligand-mediated coclustering of the 2 receptors.9 Structurally, all ITIM-containing receptors belong to the immunoglobulin receptor superfamily and carry at least 1 consensus ITIM sequence in their cytoplasmic tail, defined as I/V/LxYxxL/V (where x represents any amino acid), in reference to the previously described ITAM (consensus sequence YxxI/Lx6-12YxxI/L).10 Whereas tyrosine phosphorylation of ITAMs provides docking sites for SH2 domain–containing Syk and the structurally-related protein-tyrosine kinase Zap70 in immune cells, tyrosine phosphorylation of ITIMs provides docking sites for SH2 domain–containing lipid or protein-tyrosine phosphatases (PTPs), including SHIP-1, Shp1, and Shp2. The canonical mode of action of ITIM-containing receptors is positioning phosphatases in close proximity to ITAM-containing receptors such that the phosphatases are able to dephosphorylate and inactivate key components of the ITAM-containing receptor signaling pathway (Figure 2).
Classical inhibitory function of ITIM-containing receptors. The inhibition of ITAM-containing receptor signaling through the recruitment of the Src homology 2 (SH2) domain-containing protein-tyrosine phosphatases Shp1 and Shp2, or SH2 domain-containing inositol 5’-phosphatase 1 Ship1. Btk, Bruton's tyrosine kinase; DAG, diacylglycerol; ER, endoplasmic reticulum; IP3-R, inositol trisphosphate receptor; P, phosphate; PI3-K, phosphoinositide 3-kinase. Professional illustration by Patrick Lane, ScEYEnce Studios.
Classical inhibitory function of ITIM-containing receptors. The inhibition of ITAM-containing receptor signaling through the recruitment of the Src homology 2 (SH2) domain-containing protein-tyrosine phosphatases Shp1 and Shp2, or SH2 domain-containing inositol 5’-phosphatase 1 Ship1. Btk, Bruton's tyrosine kinase; DAG, diacylglycerol; ER, endoplasmic reticulum; IP3-R, inositol trisphosphate receptor; P, phosphate; PI3-K, phosphoinositide 3-kinase. Professional illustration by Patrick Lane, ScEYEnce Studios.
A third ITIM-like motif, termed an immunoreceptor tyrosine–based switch motif (ITSM, consensus sequence TxYxxV/I),11 was subsequently described that confers activating and/or inhibitory properties to a receptor, depending on the associated signaling proteins. ITSMs were first identified in the signaling lymphocyte adhesion molecule (SLAM) CD150, which has both activating and inhibitory functions.12 SLAM family proteins recruit members of the SLAM-adaptor protein (SAP) family, an interaction that is enhanced, but not dependent on, ITSM phosphorylation.13 When SAP proteins associate with SLAM family receptors, activating signals generally predominate, through recruitment of SFKs, whereas the absence of SAP adaptors allows phosphatase recruitment to promote inhibitory signaling.13 The abundance and availability of the SAP adaptors adds a layer of complexity to ITSM-mediated control of SLAM receptor activity. ITSMs are often found in close proximity to ITIMs, facilitating interactions with SH2 domain–containing phosphatases and contributing to the inhibitory function of ITIM-containing receptors.
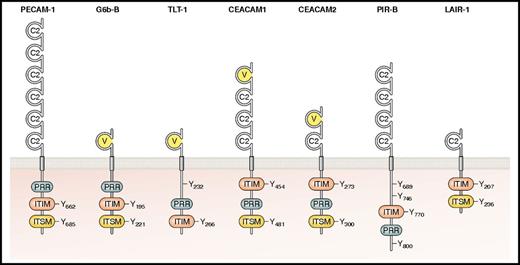
For many years, PECAM-1 (also referred to as CD31) was the only known ITIM-containing receptor in platelets.14-17 However, with the advent of proteomics and transcriptomics, several other structurally distinct ITIM-containing receptors were identified in platelets. These regulate various aspects of platelet function and include G6b-B,18,19 TREM-like transcript-1 (TLT-1),20 carcinoembryonic antigen-related cell adhesion molecule 1 (CEACAM1) and CEACAM2,21,22 and LILRB2 (also referred to as PIR-B),23 the structural features of which are summarized in Figure 3, with phenotypes of knockout (KO) and transgenic mouse models summarized in Table 1. In addition, the inhibitory collagen-binding ITIM-containing receptor LAIR-1, also shown in Figure 3, has been identified in immature megakaryocytes, but not in mature megakaryocytes or platelets.24 Recent work from our group25 has revealed that mice lacking LAIR-1 exhibit a mild thrombocytosis and increased proplatelet formation in vitro. Interestingly, platelets produced by LAIR-1 KO mice exhibit an enhanced reactivity to collagen, despite not expressing the receptor, suggesting that effects are transmitted from megakaryocytes to platelets. Two ITSM-containing SLAM family members, CD150 and CD84, have been identified in platelets, as have the SAP family proteins SAP and EAT-2.26-28 However, investigation of CD150 and CD84 KO mouse models revealed no overall effect on hemostasis, implying that their contribution to platelet function is minimal and can be compensated for.28,29
Platelet ITIM-containing receptors. The main structural features are shown, including the extracellular IgC2-like and IgV-like domains and the main intracellular signaling motifs, namely ITIMs (consensus sequence I/V/LxYxxL/V), ITSMs (consensus sequence TxYxxV/I), and PRRs (consensus sequence PxxP) along with nonconsensus ITIM/ITSM-like tyrosine residues. All receptors have been described in platelets except for LAIR-1, which is only found in megakaryocytes. Residues are numbered according to mature mouse peptide sequences, after cleavage of the signal peptide. Professional illustration by Patrick Lane, ScEYEnce Studios.
Platelet ITIM-containing receptors. The main structural features are shown, including the extracellular IgC2-like and IgV-like domains and the main intracellular signaling motifs, namely ITIMs (consensus sequence I/V/LxYxxL/V), ITSMs (consensus sequence TxYxxV/I), and PRRs (consensus sequence PxxP) along with nonconsensus ITIM/ITSM-like tyrosine residues. All receptors have been described in platelets except for LAIR-1, which is only found in megakaryocytes. Residues are numbered according to mature mouse peptide sequences, after cleavage of the signal peptide. Professional illustration by Patrick Lane, ScEYEnce Studios.
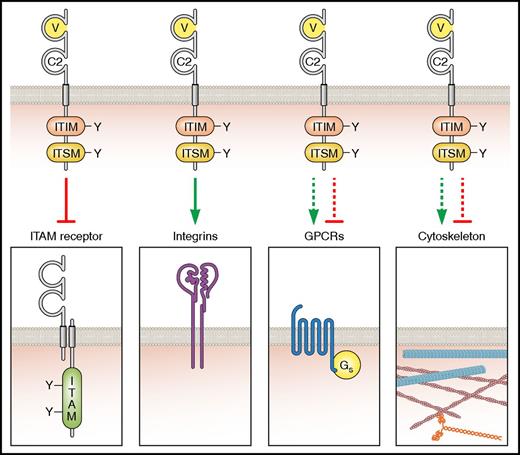
With the recent increase in the number of ITIM-containing receptors reported in platelets and their diverse functional roles in regulating platelet homeostasis, we compiled this review to summarize structural and functional features, landmark discoveries, controversies, and intriguing new findings that dispel the notion of ITIM-containing receptors as simply inhibitors of ITAM-mediated signaling (Figure 4). We focus on PECAM-1, G6b-B, and TLT-1, which together illustrate the old, the new, and the nonconventional members of this family of receptors in platelets.
Classical and putative functions of ITIM-containing receptors. Increasing evidence is implicating ITIM-containing receptors as more than just inhibitors of ITAM-containing receptors, particularly as positive regulators of integrin-mediated functions. Their potential role in both regulating cytoskeletal remodeling and G protein–coupled receptor (GPCR) signaling warrants additional investigation. Professional illustration by Patrick Lane, ScEYEnce Studios.
Classical and putative functions of ITIM-containing receptors. Increasing evidence is implicating ITIM-containing receptors as more than just inhibitors of ITAM-containing receptors, particularly as positive regulators of integrin-mediated functions. Their potential role in both regulating cytoskeletal remodeling and G protein–coupled receptor (GPCR) signaling warrants additional investigation. Professional illustration by Patrick Lane, ScEYEnce Studios.
PECAM-1: the prototype
Structure and function
PECAM-1 is expressed on the majority of nonerythroid hematopoietic cells, including platelets, monocytes, neutrophils, T cells, and B-cell subsets, an is also highly expressed on vascular endothelial cells.30 This 130-kDa receptor has a large, highly glycosylated extracellular domain comprised of 6 immunoglobulin constant 2 (IgC2)-like domains, a transmembrane domain, and a cytoplasmic tail that conveys signaling functionality. Alternative splicing of Pecam1 gives rise to a number of isoforms, with the Δ15 isoform being the most prevalent in human platelets. PECAM-1 undergoes trans homophilic interactions mediated by the 2 N-terminal IgC2-like domains D1 and D2,31,32 with particular dependence on Lys89,30 and an intracellular component encoded by exon 14 confers the capacity for heterophilic binding. A recent crystallographic study has revealed how D1 and D2 permit homophilic interactions in both cis and trans, and also how sugar moieties are accommodated at the binding interface.33 Other factors, including receptor density, glycosylation and Ca2+, can all influence PECAM-1–ligand interactions.34 This interaction is likely to be of low affinity under resting conditions, because platelets do not adhere to each other or to endothelial cells under healthy conditions, making platelet-platelet and platelet-endothelial cell interactions during thrombus formation the probable physiologically relevant context. Avidity and affinity are likely increased under these conditions. In vivo thrombosis models support this hypothesis because platelet PECAM-1 serves to limit thrombus size,35 which is discussed in detail below. However, low-affinity interactions under resting conditions cannot be discounted and may serve to strengthen inhibitory signals from PGI2 and NO. Heterophilic binding partners include the integrin αVβ336 and neutrophils CD17737 and CD38,38 which are reportedly relevant in the context of inflammation and cancer.
Adjacent to the transmembrane domain of human PECAM-1 on the cytosolic side is a palmitoylation site (C595) followed by a proline-rich region (PRR), ITIM (Y663), and ITSM (Y686); C594, Y662 and Y685, respectively, in murine PECAM-1 (Figure 3; Table 2). Ligand engagement results in tyrosine phosphorylation of the ITIMs by SFKs, notably Lyn in platelets,39 leading to recruitment of Shp1 and Shp2. The structure, phosphorylation, and accessibility of these regions to binding partners are influenced by interactions with the plasma membrane.40,41
The primary function of platelet PECAM-1 is to inhibit platelet activation and thrombosis at sites of vascular injury by attenuating signaling from the ITAM-containing receptor complex GPVI-FcR γ-chain when it engages collagen. This inhibition can, however, be overcome with high concentrations of GPVI agonists.42 Although less general and less robust than endothelial-derived PGI2 and NO,43 the inhibitory effect of PECAM-1, together with that of other ITIM-containing receptors, is likely to be highly relevant in the context of atherosclerosis, when loss of the healthy endothelium results in a reduction in the local concentration of both of these soluble mediators. In addition to its recruitment of Shp1 and Shp2 to attenuate activating signals, there is evidence that PECAM-1 can sequester activating SH2 domain–containing proteins, including SFKs and the lipid kinase phosphatidylinositol 3-kinase.44 PECAM-1 also contributes to integrin αIIbβ3 outside-in signaling,45 and to GPIbα internalization in an αIIb-dependent manner.46
Our knowledge of how PECAM-1 modulates signaling and cell activity underpins much of what we know about ITIM-containing receptor signaling in platelets and has expedited the characterization of new members of this family in this biological context.
Mouse models
Studies that use PECAM-1 KO (Pecam1−/−) mice have greatly enhanced our understanding of the physiological functions of PECAM-1. Platelets isolated from Pecam1−/− mice display potentiated aggregation and Ca2+ release and secretion in response to collagen, the GPVI-specific ligand collagen-related peptide (CRP), and thrombin.17,43 Paradoxically, platelets from Pecam1−/− mice spread less well on a fibrinogen-coated surface and exhibit reduced clot retraction compared with control platelets, suggesting that PECAM-1 is a positive regulator of αIIbβ3-mediated outside-in signaling.45 However, the mechanism underlying this potentiation of integrin-mediated responses in platelets remains unclear, and only a reduction in focal adhesion kinase phosphorylation has been shown. The observed phenotype could therefore simply be due to upregulation of compensatory mechanisms that limit platelet spreading on fibrinogen in the absence of PECAM-1. Additional work is needed to determine the exact role of PECAM-1 in regulating αIIbβ3 signaling and function.
These changes in platelet reactivity result in enhanced thrombus formation after laser- and ferric chloride–induced injury in the mesenteric arterioles and carotid arteries of mice, respectively, with both onset of thrombus formation and thrombus stability being increased.35 The use of bone marrow chimeric mice proved this to be primarily due to loss of platelet, rather than endothelial, PECAM-1.35 In contrast, tail bleeding times were increased in Pecam1−/− mice due to a loss of endothelial PECAM-1.47 This suggests that PECAM-1 has both positive and negative roles in thrombosis and hemostasis, respectively, dependent on the lineage of cell expression. It should be noted that a separate study reported no difference in thrombus formation or bleeding between wild-type and Pecam1−/− mice after photochemical-induced injury of arterioles and venules and tail-bleeding assay.48 However, there is little if any collagen exposure in this thrombosis model; instead, it is predominantly dependent on reactive oxygen species–mediated damage of endothelial cells.49 In contrast, ferric chloride injury results in endothelial denudation and exposure of the basement membrane, therefore increasing reliance on GPVI-mediated platelet activation.50 The lack of standardization and variability between different models likely accounts for the discrepancies between studies,51 but also highlights the specialized role of PECAM-1 in these processes.43 Regardless of these controversies, the evidence clearly demonstrates a vital role for PECAM-1 in regulating platelet activation and function, involving both canonical and noncanonical ITIM-mediated signaling mechanisms.
With regards to platelet turnover, Pecam1−/− mice have normal platelet counts,47,52 but exhibit a delayed recovery after antibody-mediated platelet depletion due to impaired platelet production from megakaryocytes in the bone marrow.52 This impairment is attributed to a lack of polarity of the leading edge of migrating megakaryocytes, mediated by the polarized compartmentalization of stromal-derived factor-1α chemokine receptor CXCR4, and increased adhesion to extracellular matrix proteins in vitro.53 As a consequence, PECAM-1–deficient megakaryocytes exhibit aberrant spatial distribution in the bone marrow after antibody-mediated platelet depletion.52
The biological functions of PECAM-1 extend beyond that of simply regulating platelet and megakaryocyte function. Numerous studies have highlighted the importance of PECAM-1 in a broad range of cell types, including endothelial, hematopoietic, and immune cells,54 all of which contribute to the overall phenotype of Pecam1−/− mice.
G6b-B: more than just a platelet inhibitor
Structure and function
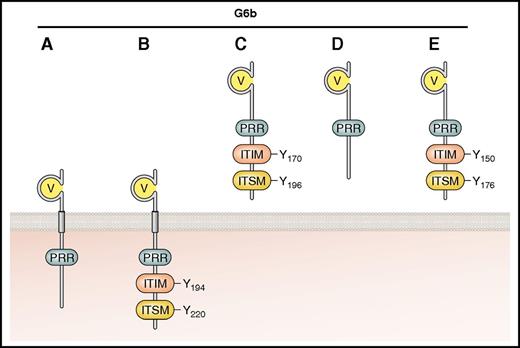
The identification of G6b-B came almost a decade after that of PECAM-1,55 and establishing its role in platelet function took an additional 10 years. Restricted to platelets and megakaryocytes, G6b-B is one of a number of predicted variants (A-E) that arise from differential splicing (Figure 5). These are either transmembrane-containing (G6b-A and -B) or potentially secreted (G6b-C, -D, and -E) isoforms.56 G6b-A and -B are both expressed in human platelets and have identical extracellular and transmembrane domains, but only the B isoform contains the consensus ITIM and ITSM. Two other isoforms, G6b-F and -G, have been predicted, but are likely not expressed because they contain intronic sequences within an alternative C-terminus.
Established and putative isoforms of human G6b. The main structural features are shown, including the IgV domain, ITIM, ITSM, and PRR. G6b-A and -B contain transmembrane regions and are therefore represented as surface receptors. G6b-C, -D, and -E, identified in transcriptome analysis but not as expressed protein, are predicted to be secreted because they lack the transmembrane domain. Residues are numbered according to mature human peptide sequences, after cleavage of the signal peptide. Professional illustration by Patrick Lane, ScEYEnce Studios.
Established and putative isoforms of human G6b. The main structural features are shown, including the IgV domain, ITIM, ITSM, and PRR. G6b-A and -B contain transmembrane regions and are therefore represented as surface receptors. G6b-C, -D, and -E, identified in transcriptome analysis but not as expressed protein, are predicted to be secreted because they lack the transmembrane domain. Residues are numbered according to mature human peptide sequences, after cleavage of the signal peptide. Professional illustration by Patrick Lane, ScEYEnce Studios.
All G6b isoforms have a single variable-type Ig-like (IgV) domain and are N-glycosylated (1 predicted site in humans and 2 in mice).19,56,57 Human G6b-B has a classical ITIM consensus sequence at the membrane proximal tyrosine (Y194) and an ITSM (Y220) at the C-terminus (Figure 5; Table 2). Two PRRs in the juxtamembrane region may provide docking sites for SH3 domain–containing proteins, but this remains to be proven. An inhibitory role for G6b-B was supported by the observation that phosphorylated G6b-B isolated from pervanadate-treated COS-7 cells coimmunoprecipitated with both Shp1 and Shp2,56 interactions that have since been verified in both human and mouse platelets.18,19,57,58 Antibody-mediated cross-linking of G6b-B also promoted Shp1 recruitment and attenuation of CRP- and ADP-induced platelet aggregation and Ca2+ release.18
In vitro assays showed G6b-B to bind the physiological anticoagulant heparin with a predicted affinity of 30 nM,59 which is comparable to the heparin-antithrombin III interaction,60 assuming a 15-kDa average molecular weight for heparin. This interaction is unexpected because G6b-B lacks a heparin-binding consensus sequence.59,61,62 However, the ectodomain of G6b-B has a theoretical isoelectric point >10, most likely giving it a positive charge at neutral pH, which may account for the interaction with highly negatively charged heparin. Heparin is released by mast cells in the subendothelial matrix and interacts with a variety of proteins, including plasma proteins, receptors, and cytokines, to modulate biological and physiological processes. The best-known function of heparin is as a cofactor of antithrombin III, conferring a 1000-fold increase in its inhibitory function of thrombin.63 The physiological significance of the interaction of heparin with G6b-B remains to be determined.
Mouse models
With the exception of glycosylation, both mouse and human G6b-B are very similar in terms of expression, amino acid sequence (71% identity, 79% similarity), tyrosine phosphorylation, and binding of Shp1 and Shp2.57 To determine the physiological function of G6b-B, constitutive and conditional KO mouse models (G6b−/− and Pf4-Cre+;G6bfl/fl, respectively) were generated.57,64 Both were healthy, fertile, and viable, with no overt growth or developmental abnormalities. However, in line with in vitro observations,65 an inhibitory role for G6b-B in regulating signaling from GPVI-FcR γ-chain and the hemi-ITAM–containing podoplanin receptor CLEC-2 was found.57
One of the most striking and unique features of G6b−/− mice is the dramatic reduction in platelet counts. This could be explained by a combination of reduced platelet recovery after antibody-mediated platelet depletion, which is supported by a reduction in G6b−/− megakaryocyte proplatelet formation, and enhanced platelet clearance due to platelets being preactivated and having surface antibodies bound, targeting them for destruction.57 Together, these defects contributed to a 77% drop in the platelet count. It is unlikely that these effects were due to the loss of G6b-B expression in other cell types because G6b-B is highly lineage specific (supplemental Figure 1, available on the Blood Web site).66 This is supported by the fact that G6b constitutive and conditional KO mouse models exhibit the same phenotype.57,64 Platelet surface receptor levels were also altered to varying degrees in G6b−/− mice, particularly GPVI, which was reduced by 82% relative to platelets from control mice. This was most likely attributable to increased receptor shedding mediated by the metalloproteinase ADAM10, which was increased by 36% in platelets from G6b−/− mice.
Collectively, the platelet defects exhibited by G6b−/− mice culminated in increased bleeding rather than a prothrombotic phenotype that would be expected when deleting an inhibitor of platelet activation. Reduced platelet counts alone cannot fully explain the bleeding diathesis, because it has previously been shown that an 80% to 90% reduction in platelet count has only mild bleeding and thrombotic consequences in mice.67 However, when combined with reduced reactivity to collagen and thrombin, as is the case in G6b−/− mice, then increased bleeding is the outcome. Collagen was still able to elicit a reduced aggregation and adenosine triphosphate secretion response due to the increased avidity and signaling through simultaneous binding of GPVI and the integrin α2β1, whereas signaling via CLEC-2 was enhanced, thus validating G6b-B as a negative regulator of ITAM-containing receptor signaling. This was further demonstrated by using Gp6+/−G6b+/− mouse platelets, which hyperresponded to GPVI agonists compared with platelets from Gp6+/− mice that expressed comparable levels of GPVI.57 Interestingly, antibody-mediated depletion of CLEC-2 in addition to deletion of Gp6 only partially rescued the G6b−/− phenotype,57 suggesting that G6b-B has additional undefined functions in megakaryocytes and platelets, independent of its inhibitory effect on ITAM-containing receptor signaling. One such function is to positively regulate integrin-mediated spreading, identified by the reduced ability of G6b−/− megakaryocytes to spread on fibrinogen, collagen, and fibronectin, and thrombin-stimulated G6b−/− platelets to spread on fibrinogen.57 These studies highlight the complexity of G6b-B signaling and demonstrate a clear role for this receptor in platelet production and clearance that cannot be compensated for by other ITIM-containing receptors. It should be noted that Shp1 and Shp2 conditional KO mice partially phenocopied G6b conditional KO mice,64 further supporting the hypothesis that these SH2 domain–containing PTPs are pivotal effectors of G6b-B. In addition, the recently described G6b-B diY/F knockin mouse model, in which tyrosine (Y) residues within ITIM and ITSM were mutated to phenylalanine (F) residues, uncoupling G6b-B from Shp1 and Shp2, recapitulated the G6b−/− phenotype.68
The physiological role of G6b-B has also now been validated in humans by Melhem and coworkers,69 who characterized a family that exhibited an autosomal recessive thrombocytopenia. Whole-exome sequencing identified a nonsense mutation at the codon for residue C108 of human G6b, resulting in a stop codon (p.C108*) and protein instability in transiently transfected K562 cells.69 Patients exhibited severe thrombocytopenia, splenomegaly, an increased number of megakaryocytes, and fibrosis in bone marrow biopsies, similar to that reported in G6b KO and G6b-B diY/F mice.57,68 Interestingly, patients harboring this mutation were not reported to have a bleeding diathesis, whereas G6b−/− mice did show increased bleeding in a tail injury model, suggesting subtle differences underlie the phenotypes in humans and mice. Although the causes of these defects reported in both species can be varied, it is worth noting that mice expressing human G6b-B do not exhibit any of these defects,70 which supports the hypothesis that human and mouse G6b-B perform the same physiological functions and are critical regulators of platelet homeostasis.
Signal transduction
Considerable evidence exists to support the hypothesis that G6b-B is an important regulator of ITAM-containing receptor and integrin signaling, as well as platelet turnover. Similar to other ITIM-containing receptors, the current understanding is that G6b-B mediates its actions largely through recruitment of Shp1 and Shp2 to the plasma membrane, but there may be additional contributions from interactions with other effectors.58 In a manner akin to the sequestration of Shp2/p85 complexes by PECAM-1,44 it is possible that G6b-B may compete for effectors of ITAM-containing receptor signaling under resting conditions to prevent spurious activation signals.58 However, these interactions have yet to be validated in platelets, and the high affinity of G6b-B for Shp1 and Shp2 (nanomolar range)58 strongly suggest these interactions are likely to dominate.
Once recruited, PTPs are predicted to be activated and dephosphorylate key substrates, leading to an attenuation of tyrosine kinase signaling and cell activation. However, the stoichiometry and downstream effects are probably more nuanced. Interestingly, G6b-B is highly phosphorylated in resting platelets than in other platelet ITIM-containing receptors.19 The significance of this is not yet known, but it suggests that G6b-B may be transmitting weak inhibitory signals even under resting conditions.
The 2 PRRs in the juxtamembrane region of the cytoplasmic tail of human G6b-B may serve to provide spatiotemporal control of signal strength, onset, or duration of signaling, as seen with GPVI. A pool of active SFKs can rapidly phosphorylate the GPVI-FcR γ-chain receptor after ligand engagement. These SFKs are maintained in a primed state through the actions of the receptor-like PTP CD148,71,72 which dephosphorylates the inhibitory tyrosine residue in the C-terminal tail of SFKs. Active Lyn and Fyn are constitutively associated with the GPVI membrane–proximal PRR via their SH3 domain. This interaction is important for the kinetics of GPVI signaling,73 with loss of the PRR delaying the onset, but not extent, of signaling by GPVI.74 Intriguingly, Fyn, Src, and Syk were shown to associate with phosphopeptides corresponding to the tandem ITIM/ITSM of G6b-B,58 possibly aiding the regulation of Shp1 and Shp2 interaction and downstream signaling. However, this has yet to be confirmed in platelets.
Thus, the biological function of G6b-B extends beyond that of inhibiting ITAM-containing receptor signaling in platelets (Figure 4). Its potential for regulating platelet reactivity under resting conditions and its apparent role in platelet production, coupled with specific megakaryocyte-platelet expression (supplemental Figure 1), offer a novel therapeutic approach for managing thrombotic and hemorrhagic risk. It is likely that the elucidation of its endogenous ligand will greatly enhance our understanding of its function.
TLT-1: the nonconventional
Structure/function
Initially identified as the inhibitory analog of the triggering receptor expressed on myeloid cells-1 (TREM-1) family of immune receptors, TLT-1 is a type I single IgV-containing surface receptor with an intracellular ITIM- and ITSM-like sequence (Figure 3; Table 2).75 Although TREM-1 recruits the ITAM-containing adaptor protein DAP12 via a positive lysine residue in its transmembrane domain,76 TLT-1 lacks this residue. TLT-1 was hypothesized to negatively regulate TREM-1 activity, yet its expression in blood cells is restricted to megakaryocytes and platelets,20 which have no detectable TREM-1 by proteomics-based approaches, raising the possibility of alternative functions. TLT-1 is in fact the most abundant ITIM-containing receptor in human and mouse platelets, which are estimated to contain 14 200 and 154 769 copies per platelet by quantitative proteomics, respectively (Table 3).26,27 However, unlike other platelet ITIM-containing receptors, TLT-1 is localized specifically in α-granules in resting platelets, and is upregulated on the surface after thrombin-mediated activation.20 Surprisingly, despite containing an ITIM and a nonconsensus ITSM (Table 2; Figure 3), TLT-1 was found to have activating functions in a transiently transfected RBL-2H3 mast cell line, which showed enhanced FcεRI-induced Ca2+ mobilization,77 whereas in platelets, antibody-mediated blocking of TLT-1 inhibits aggregation.78
Platelet activation with thrombin results in the shedding of the ectodomain of TLT-1, referred to as soluble TLT-1 (sTLT-1), which is a competitive inhibitor of TREM-1 and has anti-inflammatory properties.79 Indeed, sTLT-1 levels were substantially elevated in plasma from septic patients compared with healthy individuals, correlating with disseminated intravascular coagulation associated with sepsis.80 Recombinant protein comprised solely of the ectodomain of TLT-1 was found to augment platelet aggregation and also to bind fibrinogen, suggesting fibrinogen is the physiological ligand of membrane-bound TLT-1.80
Signal transduction
Phosphorylation of Y230 in the ITSM-like domain and Y266 in the ITIM of human TLT-1 is required for downstream signaling, the latter being critical for Shp2 recruitment in transiently transfected, pervanadate-treated RBL-2H3 cells.77 An interaction with Shp1 was demonstrated in transiently transfected HEK293 cells on discovery of the receptor,75 but this was not verified by others.77,80 Shp1 and Shp2 interactions have not been validated in platelets, but pull-down assays that use the cytoplasmic tail of the receptor did show an interaction with the ezrin/radixin/moesin (ERM) proteins in HEK293 and COS7 cells. The moesin interaction was also identified in human platelets by immunoprecipitation of full-length TLT-1.80 The ERM family links membrane proteins to the actin cytoskeleton and has important roles in lymphocyte activation and cytoskeleton remodeling during migration.81 Interestingly, TLT-1 contributes to early actin polymerization,82 indicating that ERM interaction may help to promote platelet activation. Thus, signaling via TLT-1 is distinct to that of other platelet ITIM-containing receptors.
Mouse models
The KO mouse model of TLT-1 (Treml1−/−) revealed a critical role for this receptor in dampening the inflammatory response and facilitating platelet aggregation at sites of vascular injury.80 This work confirmed an activating role for TLT-1 in platelets, notably aggregation responses to thrombin, collagen, ADP, and U46619 were reduced, as was binding to fibrinogen, with a concomitant increase in tail bleeding times.80 This function is partially mediated by fibrinogen binding and TLT-1 linking to the platelet cytoskeleton, and presumably also by facilitating activation signals through an undefined mechanism. TLT-1–deficient mice also exhibit a 20% reduction in platelet count, suggesting a role in platelet production and/or clearance.80 Lipopolysaccharide (LPS)-treated Treml1−/− mice developed higher plasma levels of TNF and d-dimer than control mice and were more likely to succumb to LPS challenge, correlating with elevated levels of sTLT-1 in septic patients.80 Despite the major advances in our understanding of the pathophysiological functions of TLT-1, several key questions remain, including the functional role of TLT-1 in platelets and megakaryocytes, how it signals, and whether platelet TLT-1 regulates leukocyte localization and function.
Other ITIM-containing receptor contributors
In addition to PECAM-1, G6b-B, and TLT-1, several other ITIM-containing receptors have been shown to regulate platelet activation and thrombus formation (Table 1). CEACAM1 and CEACAM2 are expressed on a variety of tissues, including platelets (supplemental Figure 1),66 and get upregulated on the surface of activated platelets, suggesting intracellular pools.21,22 Findings from KO mouse models showed that both CEACAM1 and CEACAM2 attenuate ITAM receptor–mediated responses, presumably via Shp1 and Shp2, but paradoxically positively regulate integrin-mediated responses,21,22,83,84 a recurring theme for platelet ITIM-containing receptors. Larger and more stable thrombi after ferric chloride injury in vivo was also demonstrated in CEACAM1- and CEACAM2-deficient mice, indicating that the net functions of CEACAM1 and CEACAM2 is to limit thrombus growth. Platelet counts were normal in both CEACAM1- and CEACAM2-deficient mice, suggesting that they are not required for platelet production and/or turnover under normal conditions. It would be interesting to determine whether deletion of both receptors has additive or synergistic effects on platelet activation and thrombosis phenotypes observed in single KO mice. However, the close proximity of the Ceacam1 and Ceacam2 genes on chromosome 7 makes generation of double-deficient mice technically challenging.85 Controversially, CEACAM1 is not detected in human platelets by proteomics-based approaches and at a low copy number in mouse platelets (868 copies per platelet). CEACAM2, found only in the murine genome, was also not detected in mouse platelets (Table 3).26,27,86 The reason for this discrepancy is not known, but it may simply be a reflection of low abundance, masking of peptides during mass spectrometry, or misassignment of peptides to other proteins after detection.87
LILRB2 (PIR-B) is the most recent addition to the platelet ITIM-containing receptor family, and transgenic mice lacking the intracellular tail are uniquely thrombocythemic.23 Platelet aggregation and signaling was mildly enhanced in response to CRP, again supporting a role in negatively regulating ITAM receptor signaling. It is noteworthy that PIR-B is not detected in either human or mouse platelets by proteomics-based approaches,26,27 possibly due to low abundance, but this raises questions about the platelet phenotype of PIR-B KO mice. Observed phenotypes may arise from the indirect effects of ablating PIR-B in other lineages and impacting platelet production. This in fact applies to most of the constitutive ITIM receptor KO and transgenic mouse models studied to date, because all are expressed in other lineages, with the exception of G6b-B and TLT-1, which are highly megakaryocyte/platelet-specific (supplemental Figure 1).66 Alternatively, because transcripts for all of these receptors are detected in the megakaryocyte lineage, the observed platelet phenotypes may be due to defects in platelet production, or transfer of activating signals to platelets, as is the case in LAIR1 KO mice,25 regardless of expression of the ITIM-containing receptor in platelets.
Summary
Platelets and megakaryocytes express an array of ITIM-containing receptors that vary in their abundance, structure, and functional significance. KO and transgenic mouse models have been pivotal in establishing the physiological functions of these receptors in the megakaryocyte lineage, but much remains to be explored. Despite all platelet ITIM-containing receptors interacting with Shp1 and Shp2, phenotypic analyses of mouse models are dramatically different and indicate additional roles beyond exclusively inhibiting ITAM receptor signaling (Figure 4). It is likely that there exists a spatiotemporal regulation system that governs receptor and effector availability and interaction, and that this system shapes distinct functional outputs. Shp1 and Shp2 are clearly major players in mediating the biological functions of ITIM-containing receptors in platelets, but the stoichiometry of binding and target specificity of these PTPs downstream of individual ITIM-containing receptors remains to be determined. In addition, an activatory role in αIIbβ3 signaling, a temporally distinct event from signaling by GPVI-FcR γ-chain and CLEC-2, has been described for PECAM-1, G6b-B, and CEACAM1/2. The mechanism by which these receptors facilitate integrin signaling remains to be determined. How TLT-1 acts as a positive regulator of platelet activation also has yet to be elucidated. A considerable amount of work remains to fully comprehend how ITIM-containing receptors work in concert with ITAM-containing receptors and integrins to orchestrate platelet reactivity and appropriate responses after injury, and how this may be overruled in pathological circumstances.
The online version of this article contains a data supplement.
Acknowledgments
The authors thank Alexandra Mazharian for contributing to the writing and editing of the manuscript (British Heart Foundation [BHF] Intermediate Basic Science Research Fellow [FS/15/58/31784]) and Timo Vögtle (Deutsche Forschungsgemeinschaft postdoctoral fellow [VO 2134/1-1]), Zaher Raslan (BHF postdoctoral researcher [PG/13/51/30296]), and Zoltan Nagy (BHF postdoctoral researcher [RG/15/13/31673]) for constructive comments on the manuscript. The authors also thank Zoltan Nagy for data mining the proteomic and transcriptomic data sets for information regarding tissue distribution of ITIM-containing receptors presented in Table 3 and supplemental Figure 1.
This work was supported by the Medical Research Council (studentship GBT1564; M.J.G.) and by BHF Senior Basic Science Research Fellowship FS/13/1/29894 (Y.A.S.).
Authorship
Contribution: C.H.C., M.J.G., and Y.A.S. wrote and revised the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Yotis A Senis, Institute of Cardiovascular Sciences, College of Medical and Dental Sciences, University of Birmingham, Birmingham B15 2TT, United Kingdom; e-mail: y.senis@bham.ac.uk
References
Author notes
C.H.C. and M.J.G. contributed equally to this study.