Key Points
TLI/ATS/alkylator conditioning allows engraftment without GVHD after curative MHC-mismatched BMT for murine β-thalassemia.
Recipient MDSCs generated in TLI/ATS/alkylator nonmyeloablative conditioning facilitate donor Treg recovery and graft-versus-host tolerance.
Abstract
Nonmyeloablative conditioning using total lymphoid irradiation (TLI) and rabbit antithymocyte serum (ATS) (the murine preclinical equivalent of antithymocyte globulin [ATG]) facilitates immune tolerance after bone marrow transplantation (BMT) across major histocompatibility complex (MHC) disparities and may be a useful strategy for nonmalignant disorders. We previously reported that donor effector T-cell function and graft-versus-host disease (GVHD) are regulated via recipient invariant natural killer T-cell (iNKT) interleukin-4–driven expansion of donor Foxp3+ naturally occurring regulatory T cells (Tregs). This occurs via recipient iNKT- and STAT6-dependent expansion of recipient myeloid dendritic cells (MDCs) that induce contact-dependent expansion of donor Treg through PD-1/PD ligand signaling. After TLI/ATS + BMT, Gr-1lowCD11c+ MDCs and Gr-1highCD11cneg myeloid-derived suppressor cells (MDSCs) were enriched in GVHD target organs. We now report that the recovery of both recipient MDSCs (P < .01) and MDCs (P < .01) is significantly increased when the alkylator cyclophosphamide (CTX) is added to TLI/ATS conditioning. In a BALB/c → B6 lethal GVHD model, adoptive transfer of MDSCs from TLI/ATS/CTX-conditioned recipients is associated with significantly improved GVHD colitis and survival (P < .001), conversion of MDSCs to PD ligand–expressing MDCs, and increased donor naturally occurring Treg recovery (P < .01) compared with control treatment. Using BALB/c donors and β-thalassemic HW-80 recipients, we found significantly improved rates of engraftment and GVHD following TLI/ATS/CTX compared with TLI/ATS, lethal or sublethal total body irradiation/ATS/CTX, or CTX/ATS conditioning. These data provide preclinical support for trials of TLI/ATG/alkylator regimens for MHC-mismatched BMT for hemoglobinopathies. The data also delineate innate immune mechanisms by which TLI/ATS/CTX conditioning may augment transplantation tolerance.
Introduction
Allogeneic hematopoietic cell transplantation (HCT) from major histocompatibility (MHC) mismatched donors provides curative HCT for the majority of patients who lack matched sibling donors.1-5 The global need for such alternative donor HCT options is particularly significant among patients with hemoglobinopathies who face race-associated disparities in donor availability in unrelated donor registries.6 MHC-mismatched HCT, particularly from haploidentical parental donors, would allow early curative HCT for the majority of hemoglobinopathy patients. Graft rejection (a result of poor host-versus-graft [HVG] immune tolerance) and graft-versus-host disease (GVHD) (poor graft-versus-host [GVH] immune tolerance) remain the most significant obstacles to MHC-mismatched HCT for nonmalignant disorders.7-13 Thus, strategies to improve bidirectional (HVG←→GVH) immune tolerance are of significant relevance to MHC-mismatched HCT for these disorders.
We recently reported a successful clinical conditioning regimen for alternative donor HCT in severe aplastic anemia14 using total lymphoid irradiation (TLI), antithymocyte globulin (ATG), and cyclophosphamide (CTX). In a murine model of β-thalassemia (β-thal) characterized by a disease-associated engraftment barrier, we demonstrate that TLI/ATS/CTX conditioning allows robust engraftment without GVHD after MHC-mismatched bone marrow transplantation (BMT).
We have previously shown in a C57BL/6 (B6) (H-2b) donor → BALB/c (H-2d) recipient mouse model of nonmyeloablative BMT that TLI/ATS conditioning creates a Th2-polarized immune milieu that enables recipient invariant natural killer T cells (iNKTs) to augment expansion of donor CD4+CD25+Foxp3+ regulatory T cells (Tregs), across MHC barriers.15 The finding that donor Treg expansion can be driven through iNKT-derived interleukin-4 (IL-4) was later replicated in a total body irradiation (TBI) conditioning model.16 Our group recently elucidated a more specific mechanism, demonstrating that iNKT- and STAT6 signaling-dependent regulatory CD11b+Gr-1lowCD11c+ MDCs maintained by submyeloablative TLI but not by TBI induce donor Foxp3+ Treg proliferation through PD-1/PD-ligand signaling.17
In these studies, we found loss of GVHD protection with administration of anti-Gr-1 (Ly6G/C) depletive antibody (clone RB6-8C5) during conditioning. RB6-8C5 depleted CD11b+Gr-1highCD11cneg MDSCs (A.S. and A.B.P., unpublished observation), a second immature myeloid subset enriched after TLI/ATS.17 MDSCs can suppress the activity of T, B, and natural killer cells,18,19 and ex vivo expanded donor-type MDSCs have been shown to regulate GVHD.20 Although other groups have reported poor dendritic cell (DC) depletion using RB6-8C5,21,22 we unexpectedly noted efficient depletion of recipient MDCs.17 Because data exist that regulatory MDCs can develop in Th2 polarized milieux,23 we tested the hypotheses that recipient MDSCs can convert to MDCs, augment donor Treg recovery, and thereby regulate GVHD. MDSCs adoptively transferred from TLI/ATS/CTX-conditioned B6 recipients into an MHC-mismatched lethal GVHD model regulated colitis and significantly improved survival. Using congenic transfers and MDC depletion, adoptively transferred MDSCs were shown to convert to regulatory PD ligand–expressing MDCs; this conversion was found to be required for enhanced donor Treg recovery after BMT. PD-1–deficient donor BMT into TLI/ATS/CTX-conditioned recipients abrogated donor Treg but not CD4 effector T-cell proliferation and decreased donor Treg recovery.
Cumulatively, these data define specific and novel mechanisms through which recipient MDSC augmented by nonmyeloablative conditioning can maintain GVH immune tolerance. The data also preclinically identify TLI/ATG + alkylator-based conditioning as a promising reduced toxicity regimen to investigate in MHC-mismatched HCT for hemoglobinopathies in humans.
Materials and methods
Mice
Wild-type (WT) (CD45.2+) and Ly5.2/CD45.1+ congenic BALB/c (H-2d) and B6 (H-2b) and CD45.2+ CD11c-human diphtheria toxin receptor (hDTR) B6 breeders were purchased from Jackson Laboratories (Bar Harbor, ME); WT and β-thal+/− B6-histocompatible HW-80 (H-2b)24 breeders were gifts from the late D. Persons (St. Jude). Animals were treated and monitored according to a St. Jude Institutional Animal Care and Use Committee–approved protocol. PD-1−/− breeders were the kind gift of Dr. Lieping Chen (Yale) via Dr. Defu Zeng (City of Hope), bred in our facilities at St. Jude and at the University of Miami.
Irradiation
ATS
CTX
A total of 200 mg/kg CTX (Baxter, Deerfield, IL) in 0.5 mL sterile saline was administered IP on days −7 and −6.
BMT
Recipients were administered 60 × 106 splenocytes (SPLs) + 50 × 106 bone marrow cells (BMs) from BALB/c donors IV at day 0 via lateral tail vein (LTV). SPLs were treated with 60% ammonium potassium chloride (GIBCO, Piscataway, NJ), washed, and resuspended in phosphate-buffered saline (PBS) before injection. Donor chimerism was assessed by day 28 and day 100 fluorescence-activated cell sorter (FACS) analysis of H-2Kd expression on peripheral blood mononuclear cells (PBMCs).
β-thal+/− or WT HW-80 mice24 were divided into conditioning groups. TLI groups received TLI (10 doses)/ATS (5 doses)/CTX (200 mg/kg), TLI/ATS, or control TLI/CTX. Additional groups received ATS/CTX alone or followed by single-dose TBI (300, 600, 800, or 1200 cGy) on day −1. BMT was performed as noted previously. BMT recipients were male and female (roughly 50% male per group) and age 8 to 12 weeks (minimum starting weight 25 g for TLI conditioning). Transplants were gender-matched. All females were nulliparous.
In vivo proliferation assays
WT male recipients were administered TLI/ATS/CTX followed at day 0 by BMT of 60 × 106 SPL + 50 × 106 BM from either WT or PD-1–deficient male donors IV via LTV. Before reconstitution in PBS for infusion with BM, SPLs were labeled with 10 mM cell proliferation dye eFluor 450 (eBioscience, San Diego, CA) at 37°C for 10 minutes per manufacturer’s protocol, quenched 3× with 50 mL ice-cold RPMI containing 10% fetal bovine serum (5 minutes), and washed by spin at 4°C, 400g for 5 minutes. At day 7 after BMT, GVHD target organs of recipients were harvested and single-cell suspensions of splenocytes prepared from each recipient separately. Cells were incubated with anti-CD16/CD32 cocktail (clone 2.4G2, BD Pharmingen, San Diego, CA) for 15 minutes before staining with the following antibodies: PerCP-Cy5.5 anti-mouse TcRβ (clone H57-597), PE-Cy7 anti-mouse CD4 (clone GK1.5), FITC anti-mouse H-2Kb (clone AF6-88.5), PE anti-mouse CD25 (clone PC61), allophycocyanin (APC)-Cy7 anti-mouse CD8 (clone 53-6.7), fixed, and permeabilized using the Foxp3 fixation/permeabilization buffer kit (Biolegend, San Diego, CA) per manufacturer’s instructions. Permeabilized cells were incubated with APC anti-mouse/rat FOXP3 (clone FJK-16s) or isotype control antibody rat IgG2a (clone eBR2a) (both from eBioscience) at 20°C for 30 minutes. Data were acquired on a 4-laser LSR-II cytometer (BD Instruments, San Jose, CA) and analyzed with FlowJo v9 (TreeStar, Eugene, OR), with proliferation gating using the proliferation function of FlowJo. LIVE/DEAD Fixable Aqua Stain (LDA) (Life Technologies, Carlsbad, CA) was used for dead cell exclusion in all analyses.
Flow cytometry
FcR-γII/III was blocked beginning 5 minutes before antibody with anti-CD16/CD32 (2.4G2, BD Pharmingen) at 4°C. Other staining reagents included the following fluorophores and antibody specificities: FITC H-2Kd (clone SF1-1.1), PE CD1d (1B1), PE CD25 (PC61) (BD Bioscience, San Jose, CA); PerCP-Cy5.5 TCRβ (H57-597), PE-Cy7 CD4 (GK 1.5), APC-Cy7 CD8 (clone 53-6.7), APC-Cy7 B220 (RA3-6B2), PerCP-Cy5.5 CD11b (M1/70), APC CD11c (HL3), FITC B220 (RA3-6B2), PE-Cy7 CD11b (M1/70), PerCP-Cy5.5 CD11c (N418) (Biolegend); APC Foxp3 (FJK-16S), e450 Gr-1 (RB6-8C5), e450 TCRβ (H57-597), e450 CD45.1 (A20), e450 CD25 (eBio 3C7), PE CD80 (16-10A1), PE CD86 (GL1), PE CD124 (mIL4R-MI), PE CD54 (ICAM-1) (3E2), PE CD252 (OX40L) (RM134L), PE H-2Kb (SF1-1.1), PE F4/80 (BM8), APC CD115 (AFS98), biotin PD-L1 (1-111A), biotin PD-L2 (clone 122), biotin PD1 (J43), PE CD40 (1C10), APC IAb (AMS-32.1) (eBioscience); goat anti-rabbit PE (Southern Biotech, Birmingham, AL), and LDA (Life Technologies).
Recipient MDSC adoptive transfer
B6 mice received TLI/ATS/CTX and BMT from BALB/c donors. At day 7, single-cell suspensions were prepared from recipient spleens, ammonium potassium chloride–treated, stained, and H-2Kd-negB220negCD11b+Gr-1high MDSCs sorted to >97% purity using a 4-laser BD FACS ARIA II (BD Instruments). MDSCs or vehicle control were infused IV via LTV into B6 recipients given 1000 cGy TBI 18 to 24 hours before secondary BMT (107 BM + 107 SPL) from BALB/c donors. In CD11c-hDTR transgenic MDSC transfers, at day 3 post-BMT, adoptive recipients received either diphtheria toxin (8 ng/g body mass) (SIGMA, Piscataway, NJ) or PBS vehicle IP.
Definition and calculation of engraftment
For all BMT experiments in this study, engraftment was defined by classic murine transplant criteria as >10% donor-specific class 1 antibody staining in at least 2 of 3 distinct lineages measured (T, B, and myeloid). Mean percentage donor chimerism (Figure 2B) was calculated for each lineage using data for all survivors in the treatment group at the specified time point (day 28 or day 100). Cumulative incidence of engraftment (Table 1) was calculated by including all recipients with engraftment at day 100. Primary nonengraftment was defined as lack of engraftment at day 28, and secondary graft rejection as engraftment at day 28 with loss of engraftment by day 100.
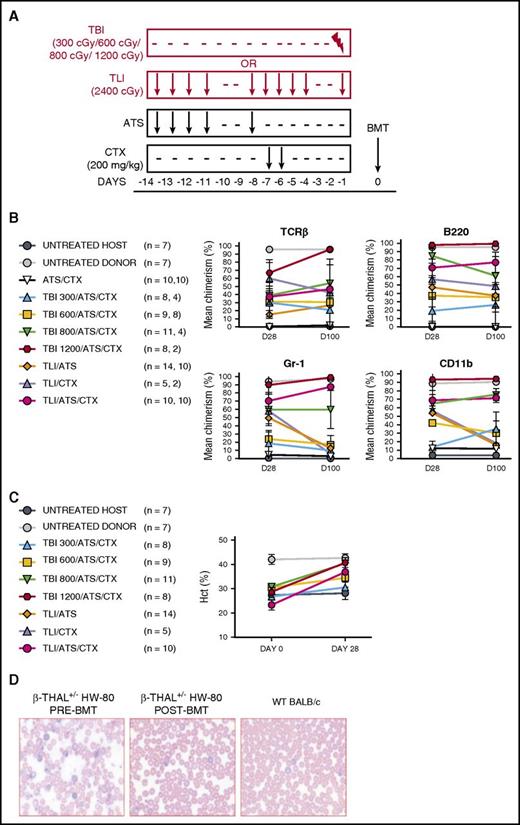
Host-versus-graft immune tolerance and disease correction following TLI/ATS/CTX compared with other conditioning before MHC-mismatched allogeneic BMT for β-thal. (A) Experimental design for comparison of nonmyeloablative TLI/ATS, ATS/CTX, and nonmyeloablative and myeloablative TBI/ATS/CTX conditioning in β-thal+/− HW-80 recipients. BMT = 50 × 106 bone marrow + 60 × 106 spleen cells from WT BALB/c (H-2d) donors. (B) Mean ± SEM donor chimerism (%) at D28 and D100 by FACS analysis of gated live lymphoid lineage (B220+, TCRαβ+) cells (top) and myeloid lineage (Gr-1/Ly6+, CD11b/Mac-1+) cells (bottom) in peripheral blood of β-thal+/− HW-80 recipients of WT BALB/c BMT. Data are cumulative (n = 28 total experiments). N values are the number of recipients analyzed at D28 (first value) and D100 (second value), respectively, based upon cumulative survival of recipients at each time point. (C) Mean ± SEM peripheral blood hematocrit (%) at days 0 pre-BMT and 28 post-BMT in β-thal+/− HW-80 recipients in panel B. N represents the total number of starting mice in each group at day 0. (D) Representative photomicrograph of Giemsa-stained β-thal+/− HW-80 recipient peripheral blood film before BMT (left) and at D28 following TLI/ATS/CTX conditioning and BMT (middle) from its WT BALB/c donor (right).
Host-versus-graft immune tolerance and disease correction following TLI/ATS/CTX compared with other conditioning before MHC-mismatched allogeneic BMT for β-thal. (A) Experimental design for comparison of nonmyeloablative TLI/ATS, ATS/CTX, and nonmyeloablative and myeloablative TBI/ATS/CTX conditioning in β-thal+/− HW-80 recipients. BMT = 50 × 106 bone marrow + 60 × 106 spleen cells from WT BALB/c (H-2d) donors. (B) Mean ± SEM donor chimerism (%) at D28 and D100 by FACS analysis of gated live lymphoid lineage (B220+, TCRαβ+) cells (top) and myeloid lineage (Gr-1/Ly6+, CD11b/Mac-1+) cells (bottom) in peripheral blood of β-thal+/− HW-80 recipients of WT BALB/c BMT. Data are cumulative (n = 28 total experiments). N values are the number of recipients analyzed at D28 (first value) and D100 (second value), respectively, based upon cumulative survival of recipients at each time point. (C) Mean ± SEM peripheral blood hematocrit (%) at days 0 pre-BMT and 28 post-BMT in β-thal+/− HW-80 recipients in panel B. N represents the total number of starting mice in each group at day 0. (D) Representative photomicrograph of Giemsa-stained β-thal+/− HW-80 recipient peripheral blood film before BMT (left) and at D28 following TLI/ATS/CTX conditioning and BMT (middle) from its WT BALB/c donor (right).
GVHD assessments
Animals were euthanized at either: (1) day 7 (immune studies) or (2) when moribund or at day 100 if nonmoribund (survival studies). Skin, liver, and the terminal 1 cm of descending colon were fixed in 10% formalin, cut in 4- to 5-μm sections, stained with hematoxylin and eosin, microscopically reviewed, and assigned GVHD scores.15,17 The evaluating pathologist was blinded to the experimental groups. Mean GVHD scores (Figures 1, 3, 4, and 5) and cumulative incidence of GVHD (Table 1) were calculated by combining data for all mice engrafting at day 28 and with stable engraftment at day 100 as well as for mice dying before day 100 with histopathologic evidence of GVHD (censored). Histopathologic evidence of GVHD was defined as a colon score of >1 or a cumulative score >2 (the baseline scores of myeloablated negative controls receiving BM only).
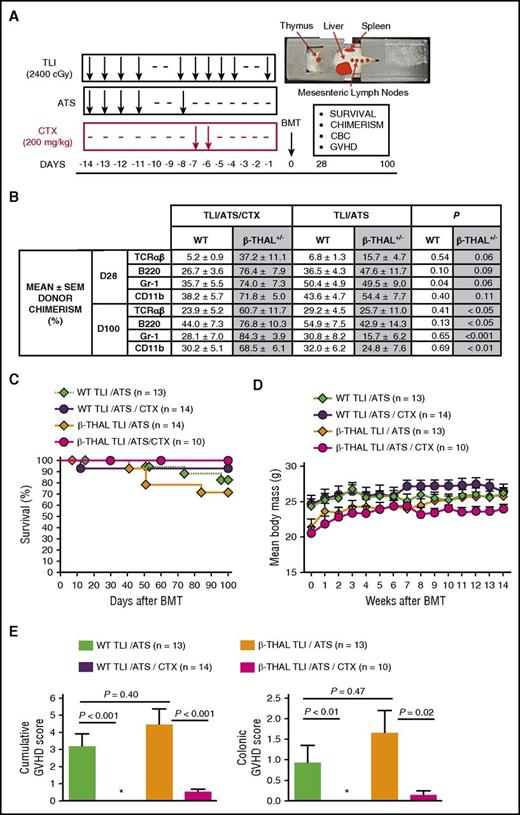
TLI/ATS/CTX overcomes engraftment and GVHD barriers following MHC-mismatched allogeneic BMT for β-thal. (A) Experimental design for TLI/ATS and TLI/ATS/CTX conditioning. Representative image (top right) of recipient mouse in lead shielding jig delineates TLI-exposed regions. Main monitored parameters in recipients of BMT from day 0 to day 100 are indicated in the text box. BMT = 50 × 106 BM + 60 × 106 spleen cells from WT BALB/c (H-2d) donors. CBC, complete blood count. (B) Mean ± standard error of the mean (SEM) donor chimerism (%) at day 28 (D28) and day 100 (D100) measured by FACS analysis of gated live subsets (TCRαβ+, B220+, Gr-1/Ly6+, CD11b/Mac-1+) in peripheral blood of WT and β-thal+/− HW-80 recipients of TLI/ATS/CTX or TLI/ATS conditioning and WT BALB/c BMT. P values given are between TLI/ATS/CTX and TLI/ATS treatment within WT or β-thalassemic (β-thal+/−) recipient groups indicated. (C) Kaplan-Meier analysis of cumulative survival (%) of WT and β-thal+/− HW-80 recipients of TLI/ATS/CTX or TLI/ATS conditioning and WT BALB/c BMT. Data are cumulative (n = 8 experiments). (D) Mean ± SEM body mass (grams) of mice engrafting by day 28 (includes those rejecting the donor graft by D100) in experimental groups shown in C. Data are cumulative (n = 8 experiments). (E) Mean ± SEM cumulative GVHD scores (left) and colon GVHD scores (right) of engrafting WT and β-thal+/−HW-80 recipients of TLI/ATS/CTX or TLI/ATS conditioning and WT BALB/c BMT. Data are cumulative (n = 8 experiments). *Mean score, 0.
TLI/ATS/CTX overcomes engraftment and GVHD barriers following MHC-mismatched allogeneic BMT for β-thal. (A) Experimental design for TLI/ATS and TLI/ATS/CTX conditioning. Representative image (top right) of recipient mouse in lead shielding jig delineates TLI-exposed regions. Main monitored parameters in recipients of BMT from day 0 to day 100 are indicated in the text box. BMT = 50 × 106 BM + 60 × 106 spleen cells from WT BALB/c (H-2d) donors. CBC, complete blood count. (B) Mean ± standard error of the mean (SEM) donor chimerism (%) at day 28 (D28) and day 100 (D100) measured by FACS analysis of gated live subsets (TCRαβ+, B220+, Gr-1/Ly6+, CD11b/Mac-1+) in peripheral blood of WT and β-thal+/− HW-80 recipients of TLI/ATS/CTX or TLI/ATS conditioning and WT BALB/c BMT. P values given are between TLI/ATS/CTX and TLI/ATS treatment within WT or β-thalassemic (β-thal+/−) recipient groups indicated. (C) Kaplan-Meier analysis of cumulative survival (%) of WT and β-thal+/− HW-80 recipients of TLI/ATS/CTX or TLI/ATS conditioning and WT BALB/c BMT. Data are cumulative (n = 8 experiments). (D) Mean ± SEM body mass (grams) of mice engrafting by day 28 (includes those rejecting the donor graft by D100) in experimental groups shown in C. Data are cumulative (n = 8 experiments). (E) Mean ± SEM cumulative GVHD scores (left) and colon GVHD scores (right) of engrafting WT and β-thal+/−HW-80 recipients of TLI/ATS/CTX or TLI/ATS conditioning and WT BALB/c BMT. Data are cumulative (n = 8 experiments). *Mean score, 0.
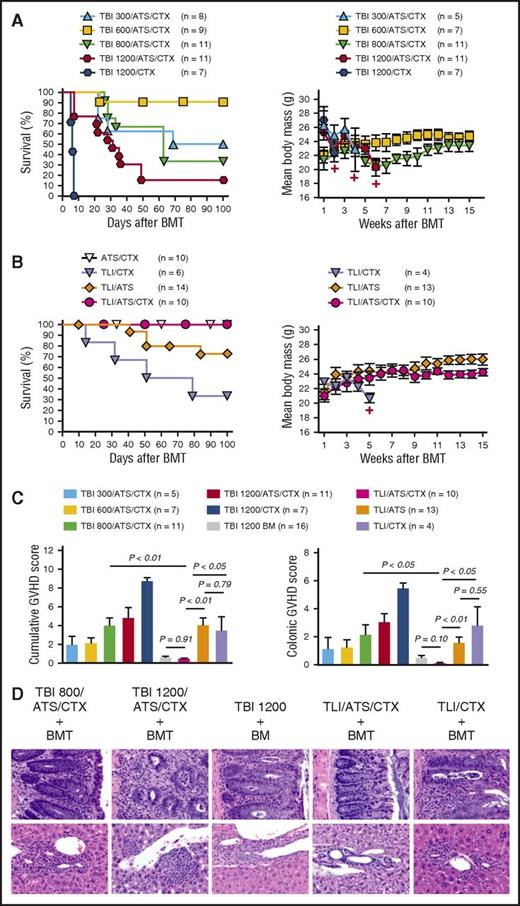
Graft-versus-host immune tolerance following TLI/ATS/CTX conditioning for MHC-mismatched allogeneic BMT for β-thal. (A) Kaplan-Meier analysis of cumulative survival (%) for all mice in each conditioning group (left) and mean ± SEM body mass (grams) of those mice engrafting by day 28 (right) among β-thal+/− HW-80 recipients of WT BALB/c BMT following TBI-containing conditioning. Data are cumulative (n = 28 experiments). Number represents cumulative dose (cGy) TBI (eg, TBI 300, 300 cGy TBI). +, Time point at which 3 or fewer survivors remain in the specified treatment group. (B) Cumulative survival (%) for all mice in each conditioning group (left) and mean ± SEM body mass (grams) of those mice engrafting by day 28 (right) among β-thal+/− HW-80 recipients of TLI-based conditioning or control ATS/CTX conditioning followed by WT BALB/c BMT. Data are cumulative (n = 28 experiments). (C) Mean ± SEM cumulative GVHD scores (left) and colon GVHD scores (right) of those mice engrafting by day 28 among BMT recipients shown in panels A and B. Data are cumulative (n = 28 experiments). (D) Representative photomicrographs of hematoxylin and eosin–stained sections of colon (40×) (top) and liver showing portal triad (40×) (bottom) from selected groups shown in panel C.
Graft-versus-host immune tolerance following TLI/ATS/CTX conditioning for MHC-mismatched allogeneic BMT for β-thal. (A) Kaplan-Meier analysis of cumulative survival (%) for all mice in each conditioning group (left) and mean ± SEM body mass (grams) of those mice engrafting by day 28 (right) among β-thal+/− HW-80 recipients of WT BALB/c BMT following TBI-containing conditioning. Data are cumulative (n = 28 experiments). Number represents cumulative dose (cGy) TBI (eg, TBI 300, 300 cGy TBI). +, Time point at which 3 or fewer survivors remain in the specified treatment group. (B) Cumulative survival (%) for all mice in each conditioning group (left) and mean ± SEM body mass (grams) of those mice engrafting by day 28 (right) among β-thal+/− HW-80 recipients of TLI-based conditioning or control ATS/CTX conditioning followed by WT BALB/c BMT. Data are cumulative (n = 28 experiments). (C) Mean ± SEM cumulative GVHD scores (left) and colon GVHD scores (right) of those mice engrafting by day 28 among BMT recipients shown in panels A and B. Data are cumulative (n = 28 experiments). (D) Representative photomicrographs of hematoxylin and eosin–stained sections of colon (40×) (top) and liver showing portal triad (40×) (bottom) from selected groups shown in panel C.
TLI/ATS/CTX compared with TLI/ATS conditioning results in enhanced generation of recipient Gr-1highCD11b+CD11cnegMDSCs and augments recovery of donor Foxp3+Treg after BMT. (A) Representative 3-dimensional plot of FLOCK centroid analysis (top) on gated H-2Kd-negB220neg cells for PBMC obtained at day 7 post-BMT from a β-thal+/− HW-80 recipient of TLI/ATS or TLI/ATS/CTX and BALB/c BMT, showing recipient MDSCs (blue, pink) and MDC (red, gold). The MDSC (lower left) and MDC tables (lower right) show frequency (%) of the indicated color plots among total analyzed cells as well as percentages for analysis of a control nonincreased population (MDSC, teal; MDC, violet). T/A, TLI/ATS; T/A/C, TLI/ATS/CTX. (B) Representative 3-dimensional plot of FLOCK centroid analysis (top) for PBMC at day 7 post-BMT from a β-thal+/− HW-80 recipient of TLI/ATS or TLI/ATS/CTX and BALB/c BMT. Percentage nTreg populations (green, gold) and 1 control nonincreased population (violet) among total analyzed cells is shown in the Treg table (bottom). (C) Mean ± SEM absolute number H-2Kd-negB220negCD11b+Gr-1highCD11cneg cells (MDSC) (left) and H-2Kd-negB220negCD11b+Gr-1lowCD11c+ cells (MDC) (right) at day 7 in spleen of recipients of TLI/ATS or TLI/ATS/CTX and WT BALB/c donor BMT. (D) Protocol for MDSC depletion during TLI/ATS/CTX conditioning. WT B6 mice received TLI/ATS/CTX and IP injection of depletive anti-Gr-1/Ly6 clone RB6-8C5 or rat IgG2a isotype control antibody, followed by WT BALB/c BMT. At day 7 after BMT, recipients were euthanized for FACS analysis of MDSC, MDC, and Foxp3+ Treg recovery and histopathologic assessment of GVHD target organs. BMT = 50 × 106 bone marrow cells + 60 × 106 spleen cells from WT BALB/c donors. (E) Mean ± SEM absolute number recipient MDSCs (left), recipient MDCs (middle), and donor Treg (right) at day 7 post-BMT in spleens of recipients of IgG2a isotype control antibody or RB6-8C5 during TLI/ATS/CTX conditioning. (F) Mean ± SEM cumulative GVHD scores for mice represented in panel E. Data are cumulative (n = 3 experiments). Representative photomicrographs of hematoxylin and eosin–stained sections of colon (×40) (left) and liver (×40) (right) from each group.
TLI/ATS/CTX compared with TLI/ATS conditioning results in enhanced generation of recipient Gr-1highCD11b+CD11cnegMDSCs and augments recovery of donor Foxp3+Treg after BMT. (A) Representative 3-dimensional plot of FLOCK centroid analysis (top) on gated H-2Kd-negB220neg cells for PBMC obtained at day 7 post-BMT from a β-thal+/− HW-80 recipient of TLI/ATS or TLI/ATS/CTX and BALB/c BMT, showing recipient MDSCs (blue, pink) and MDC (red, gold). The MDSC (lower left) and MDC tables (lower right) show frequency (%) of the indicated color plots among total analyzed cells as well as percentages for analysis of a control nonincreased population (MDSC, teal; MDC, violet). T/A, TLI/ATS; T/A/C, TLI/ATS/CTX. (B) Representative 3-dimensional plot of FLOCK centroid analysis (top) for PBMC at day 7 post-BMT from a β-thal+/− HW-80 recipient of TLI/ATS or TLI/ATS/CTX and BALB/c BMT. Percentage nTreg populations (green, gold) and 1 control nonincreased population (violet) among total analyzed cells is shown in the Treg table (bottom). (C) Mean ± SEM absolute number H-2Kd-negB220negCD11b+Gr-1highCD11cneg cells (MDSC) (left) and H-2Kd-negB220negCD11b+Gr-1lowCD11c+ cells (MDC) (right) at day 7 in spleen of recipients of TLI/ATS or TLI/ATS/CTX and WT BALB/c donor BMT. (D) Protocol for MDSC depletion during TLI/ATS/CTX conditioning. WT B6 mice received TLI/ATS/CTX and IP injection of depletive anti-Gr-1/Ly6 clone RB6-8C5 or rat IgG2a isotype control antibody, followed by WT BALB/c BMT. At day 7 after BMT, recipients were euthanized for FACS analysis of MDSC, MDC, and Foxp3+ Treg recovery and histopathologic assessment of GVHD target organs. BMT = 50 × 106 bone marrow cells + 60 × 106 spleen cells from WT BALB/c donors. (E) Mean ± SEM absolute number recipient MDSCs (left), recipient MDCs (middle), and donor Treg (right) at day 7 post-BMT in spleens of recipients of IgG2a isotype control antibody or RB6-8C5 during TLI/ATS/CTX conditioning. (F) Mean ± SEM cumulative GVHD scores for mice represented in panel E. Data are cumulative (n = 3 experiments). Representative photomicrographs of hematoxylin and eosin–stained sections of colon (×40) (left) and liver (×40) (right) from each group.
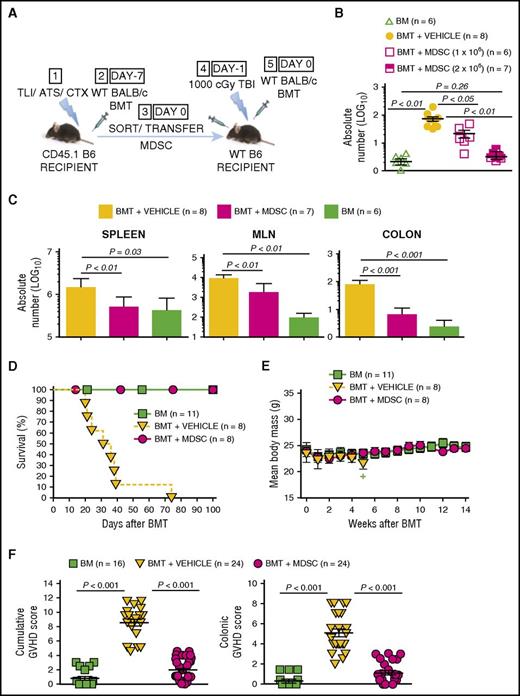
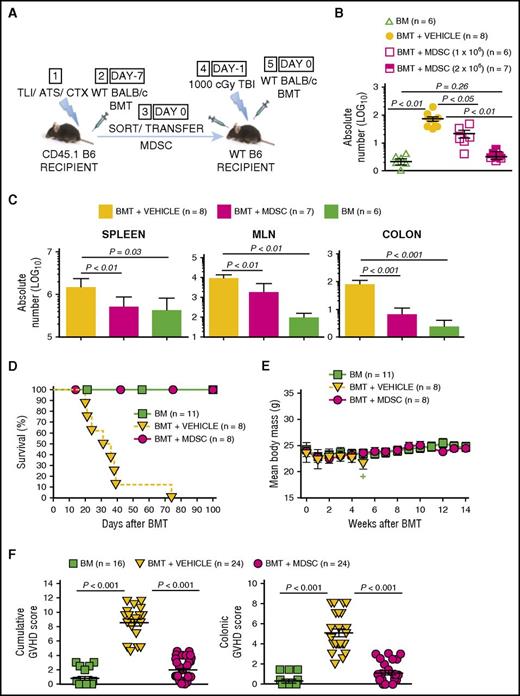
Adoptively transferred MDSCs derived from TLI/ATS/CTX-conditioned recipients prevent MHC-mismatched lethal GVHD. (A) Protocol for MDSC adoptive transfer studies: (1) CD45.1 congenic B6 mice received TLI/ATS/CTX followed by (2) WT BALB/c BMT. At 7 days following BMT, H-2Kd-negB220negCD11b+Gr-1highCD11cneg MDSCs were sorted from spleens of recipients in (2) and infused IV (3) into adoptive WT (CD45.2) B6 recipients of 1000 cGy TBI (4), followed by (5) WT BALB/c BMT. Adoptive hosts were either euthanized at day 7 for FACS analysis and histopathologic assessment of key GVHD target organs, or monitored for 100 days for survival and signs of GVHD. BMT at day −7 = 50 × 106 bone marrow cells + 60 × 106 spleen cells from BALB/c donors; BMT at day 0 = 10 × 106 bone marrow cells + 10 × 106 spleen cells from BALB/c donors. (B) Mean ± SEM absolute number (log 10) H-2Kd+TCRαβ+CD8+ donor GVHD effector cells in colons of adoptive hosts receiving BM alone (negative control), BMT + PBS vehicle (positive control), or BMT + 1 × 106 or 2 × 106 sorted recipient MDSCs. Data are cumulative (n = 3 experiments). (C) Mean ± SEM absolute number (log 10) H-2Kd+TCRαβ+CD8+ donor GVHD effector cells in spleens (left), MLN (middle), and colon (right) at day 7 post-BMT in adoptive hosts administered BMT + PBS vehicle, BMT + 2 × 106 sorted MDSCs, or BM alone. Data represent n = 3 experiments. (D) Kaplan-Meier cumulative survival (%) of adoptive hosts shown in panel A. Data are from n = 4 representative experiments. (E) Mean ± SEM body mass (g) of mice in panel D. Data are from n = 4 representative experiments. Plus sign indicates time point at which 3 or fewer survivors remain in the specified treatment group. (F) Mean ± SEM cumulative GVHD scores (left) and colon GVHD scores (right) of experimental groups shown in panel D. Data are cumulative (n = 8 experiments).
Adoptively transferred MDSCs derived from TLI/ATS/CTX-conditioned recipients prevent MHC-mismatched lethal GVHD. (A) Protocol for MDSC adoptive transfer studies: (1) CD45.1 congenic B6 mice received TLI/ATS/CTX followed by (2) WT BALB/c BMT. At 7 days following BMT, H-2Kd-negB220negCD11b+Gr-1highCD11cneg MDSCs were sorted from spleens of recipients in (2) and infused IV (3) into adoptive WT (CD45.2) B6 recipients of 1000 cGy TBI (4), followed by (5) WT BALB/c BMT. Adoptive hosts were either euthanized at day 7 for FACS analysis and histopathologic assessment of key GVHD target organs, or monitored for 100 days for survival and signs of GVHD. BMT at day −7 = 50 × 106 bone marrow cells + 60 × 106 spleen cells from BALB/c donors; BMT at day 0 = 10 × 106 bone marrow cells + 10 × 106 spleen cells from BALB/c donors. (B) Mean ± SEM absolute number (log 10) H-2Kd+TCRαβ+CD8+ donor GVHD effector cells in colons of adoptive hosts receiving BM alone (negative control), BMT + PBS vehicle (positive control), or BMT + 1 × 106 or 2 × 106 sorted recipient MDSCs. Data are cumulative (n = 3 experiments). (C) Mean ± SEM absolute number (log 10) H-2Kd+TCRαβ+CD8+ donor GVHD effector cells in spleens (left), MLN (middle), and colon (right) at day 7 post-BMT in adoptive hosts administered BMT + PBS vehicle, BMT + 2 × 106 sorted MDSCs, or BM alone. Data represent n = 3 experiments. (D) Kaplan-Meier cumulative survival (%) of adoptive hosts shown in panel A. Data are from n = 4 representative experiments. (E) Mean ± SEM body mass (g) of mice in panel D. Data are from n = 4 representative experiments. Plus sign indicates time point at which 3 or fewer survivors remain in the specified treatment group. (F) Mean ± SEM cumulative GVHD scores (left) and colon GVHD scores (right) of experimental groups shown in panel D. Data are cumulative (n = 8 experiments).
Donor CD8+ T-cell accumulation
Erythrocyte analysis
Peripheral blood was analyzed on a Forcyte 2 cytometer (Oxford Science, Oxford, CT) and Giemsa-stained smears reviewed at days 28 and 100.
FLOCK
The FLOw Clustering without K (FLOCK)25,26 computational program was accessed by registration at http://www.immport.org/immport-open/public/home/home. The .fcs files from standard Diva software were uploaded using FCSTrans (ImmPort FCS file converter).27 Centroid files and statistical comparisons were generated in FLOCK between populations across samples and graphically displayed using the 3-dimensional function.
Statistical analysis
Statistical significance in survival was assessed by log-rank test. Significance of continuous variables including mean GVHD scores, absolute cell numbers, and hematocrit was assessed by Mann-Whitney U test. P < .05 was considered significant.
Results
Addition of CTX to TLI/ATS conditioning enhances engraftment, improves survival, and reduces GVHD in β-thal recipients of MHC-mismatched BMT
We examined HVG and GVH immune tolerance after WT BALB/c donor BMT into WT and β-thal+/− HW-80 (H-2b, fully B6-histocompatible) recipients of TLI/ATS or TLI/ATS/CTX (Figure 1A). Donor cell dose was selected to mimic the total nucleated and CD3+ cell doses administered to the previously reported cohort of TLI/ATG/CTX-conditioned patients with severe aplastic anemia.14
With the exception of day 28 granulocyte chimerism, there was no significant difference in day 28 or day 100 donor chimerism between WT HW-80 recipients of TLI/ATS or TLI/ATS/CTX (Figure 1B). β-thal+/− TLI/ATS/CTX-conditioned recipients showed significantly higher day 100 chimerism than TLI/ATS recipients (TCRαβ+: P = .04; B220+: P = .04; Gr-1+: P < .001; CD11b+: P < .01) (Figure 1B). Table 1 overviews the cumulative incidence of major events after BMT by treatment group. Among recipients of TLI/ATS conditioning, 5 of 14 (35.7%) β-thal+/− (Table 1) and 0% of WT (data not shown) demonstrated either no primary engraftment by or graft rejection after day 28 (P < .05). The significant differences in cumulative incidence of engraftment (Table 1) and percentage donor chimerism (Figure 1B) between WT and β-thal+/− recipients conditioned with TLI/ATS and transplanted identically supports the notion that a disease-associated barrier to long-term HVG tolerance exists in thalassemic compared with WT recipients of MHC-mismatched BMT. This further supports the preclinical utility of this model for transplantation studies. This relative barrier to engraftment could be overcome by addition of CTX to TLI/ATS.
A total of 92.8% of WT and 100% of β-thal TLI/ATS/CTX recipients survived beyond day 100 after BMT (Figure 1C), with increasing mean body mass (Figure 1D). The single death in the WT TLI/ATS/CTX group was unrelated to BMT and without histopathologic GVHD (Figure 1E). Among β-thal recipients of TLI/ATS, 4 of 13 (30.8%) engrafting by day 28 died before day 100 (Figure 1C). Survival was significantly increased in engrafting TLI/ATS/CTX-conditioned β-thal+/− mice (P = .03) (Figure 1C), but not different between TLI/ATS- and TLI/ATS/CTX-conditioned WT recipients (P = .45) or between WT and β-thal+/− recipients of TLI/ATS (P = .40) or TLI/ATS/CTX (P = .79). Significant GVHD colitis (Figure 1E-F) was present in WT (P < .01) and β-thal+/− (P < .05, colon and cumulative) TLI/ATS recipients. Mean GVHD scores of TLI/ATS recipients engrafting at day 28 were not significantly different between WT and β-thal+/− mice (P ≥ .4, cumulative and colon GVHD scores) (Figure 1E). TLI/ATS/CTX recipients showed no histopathologic GVHD at day 100 (Figure 1E-F), despite higher multilineage donor chimerism in this group than in TLI/ATS recipients (Figure 1B). These data support the notion that addition of CTX to TLI/ATS before BMT augmented long-term GVH tolerance after BMT.
TLI/ATS/CTX facilitates stable donor engraftment and disease correction in β-thal recipients of MHC-mismatched BMT
We assessed HVG and GVH tolerance in β-thal+/− recipients of TLI/ATS, TLI/ATS/CTX, and TBI-based or ATS/CTX conditioning (Figure 2A). β-thal+/− recipients of ATS/CTX uniformly did not engraft (Table 1) but maintained recipient hematopoiesis through day 100 (Figure 2B), demonstrating that ATS/CTX is nonmyeloablative in this model. Mean day 28 multilineage donor chimerism increased between ATS/CTX and TBI/ATS/CTX groups, directly correlating with cumulative TBI dose (Figure 2B).
By day 100, β-thal+/− recipients of TLI/ATS/CTX demonstrated significantly higher donor chimerism than TLI/ATS recipients (TCRαβ+: P < .05; B220+: P < .05; Gr-1+: P < .001; CD11b+: P < .001), not significantly different from day 100 survivors of myeloablative 1200 cGy TBI/ATS/CTX + BMT (TBI 1200/ATS/CTX) (TCRαβ+: P = .33; B220+: P = .64; Gr-1+: P = .07; CD11b+: P = .07). Day 100 survivors of reduced-dose TBI conditioning (300 or 600 cGy) demonstrated significantly lower myeloid lineage donor chimerism compared with TLI/ATS/CTX (TBI 300/ATS/CTX or TBI 600/ATS/CTX: Gr-1+: P < .05; CD11b+: P < .05), despite identical CTX and ATS dose and schedule. Day 100 survivors of 800 cGy TBI/ATS/CTX (TBI 800/ATS/CTX) (submyeloablative in HW-80) had mean donor chimerism comparable to TLI/ATS/CTX recipients (TCRαβ+: P = .84; B220+: P = .88; Gr-1+: P = .65; CD11b+: P = .90) (Figure 2B).
Figure 2C shows peripheral blood mean hematocrit (%) at day 0 pre-BMT and 28 post-BMT for all engrafting groups. Untreated β-thal+/− mice had significantly lower hematocrit than BALB/c donors (P = .002). TLI/ATS/CTX + BMT significantly increased day 28 hematocrit compared with untreated β-thal+/− mice (P = .006), approximating mean hematocrit of WT BALB/c (P = .10). Engrafting TLI/ATS recipients showed a trend toward day 28 hematocrit correction (P = .07), although significantly lower than that of TLI/ATS/CTX-conditioned recipients (P = .01) or WT BALB/c (P < .001) (Figure 2C). Among TBI/ATS/CTX groups, myeloablative TBI 1200/ATS/CTX (P < .01) and high-dose submyeloablative TBI 800/ATS/CTX (P < .01) facilitated day 28 hematocrit correction compared with no treatment, but not low-dose submyeloablative TBI 300/ATS/CTX (P = .99) or TBI 600/ATS/CTX (P = .08) (Figure 2C). Figure 2D shows Giemsa-stained peripheral blood smears of a representative β-thal+/− recipient pre-BMT (Figure 2D, left panel) and after TLI/ATS/CTX + BMT (Figure 2D, middle panel).
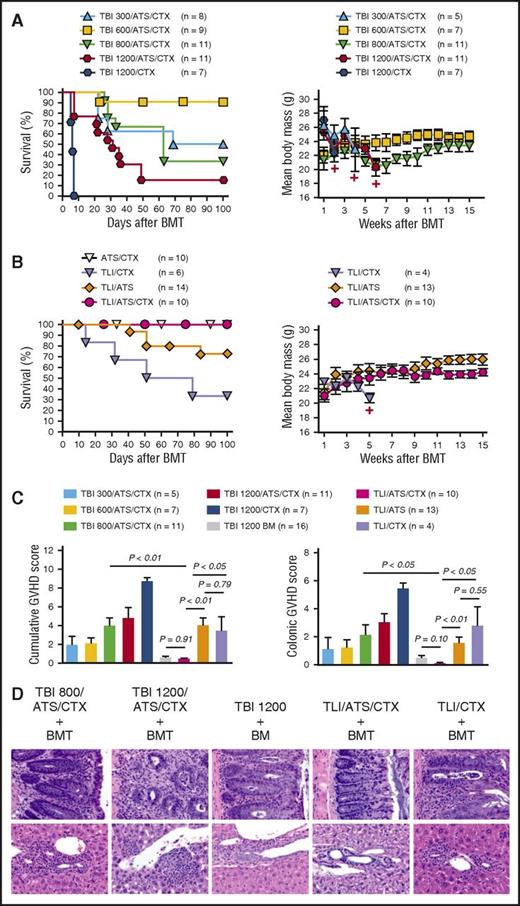
TLI/ATS/CTX enhances GVH tolerance over TBI/ATS/CTX in β-thal+/− recipients of MHC-mismatched BMT
Of β-thal+/− recipients of 1200 TBI/ATS/CTX, 81.8% died before day 60 post-BMT (Figure 3A, left panel), with weight loss (Figure 3A, right panel) and GVHD colitis (Figure 3C-D; Table 1). Controls receiving 1200 cGy TBI + CTX (1200 TBI/CTX) without ATS developed a rapidly lethal (hyperacute) GVHD (1200 TBI/CTX vs 1200 TBI/ATS/CTX: log-rank P < .01) (Figure 3A, left panel), with profound weight loss (Figure 3A, right panel) and histopathologic GVHD (Figure 3C-D). Among recipients of nonmyeloablative (300, 600, or 800 cGy) TBI + ATS/CTX, the best cumulative incidence of day 100 survival with engraftment without GVHD was ≤33.3% (Table 1), indicating poor bidirectional tolerance. Mean GVHD scores in engrafting mice were significantly higher than controls receiving 1200 cGy TBI and 50 × 106 BM alone (TBI 1200 BM only) in TBI 800/ATS/CTX (cumulative: P < .01) and TBI 600/ATS/CTX (cumulative: P < .01), but not in TBI 300/ATS/CTX (cumulative: P = .07; colon: P = .57). Colon GVHD scores were also higher than TLI/ATS/CTX recipients in TBI 800/ATS/CTX (P = .03) and TBI 600/ATS/CTX (P = .04), but not in TBI 300/ATS/CTX (P = .12).
Among TLI/ATS recipients, 71.4% survived 100 days, 92.8% engrafted, 30.8% experienced secondary graft rejection, 30.8% developed GVHD (Table 1), and mean colon GVHD scores among engrafting mice were significantly higher than both TBI 1200 BM controls (P < .05) and TLI/ATS/CTX recipients (P < .01) (Figure 3C-D). Of TLI/CTX-conditioned recipients, 83.3% engrafted, 60.0% died by day 100 with clinical (Figure 3B, right panel) and histopathologic GVHD (Figure 3C-D); 16.7% remained engrafted without GVHD at day 100 (Table 1). Cumulative GVHD scores of engrafting TLI/CTX recipients were significantly higher than those of TBI 1200 BM controls (P < .01) and TLI/ATS/CTX (P < .05) but not TLI/ATS recipients (P = .79), suggesting that either alkylator or ATS alone combined with TLI was suboptimal for establishing durable GVH tolerance.
Of TLI/ATS/CTX recipients, 100% engrafted by day 28 and 100% survived to day 100 (Figure 3B, left panel). Cumulative incidence of engraftment at day 100 was 100% and of GVHD 0% (Table 1). All recipients demonstrated donor-recipient mixed chimerism (Figure 2B) and increasing mean body mass (Figure 3B, right panel). GVHD scores in this group were not significantly increased above recipients of TBI 1200 BM alone (cumulative: P = .91; colon: P = .10) (Figure 3C-D).
TLI/ATS/CTX enhances MDSC and MDC generation in both thalassemic and WT recipients of MHC-mismatched BMT
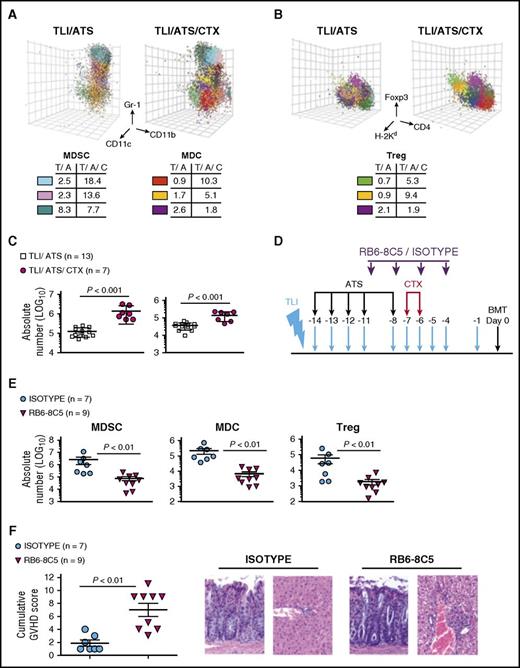
We investigated immune mechanisms by which the addition of CTX to TLI/ATS pre-BMT could augment GVHD protection beyond day 28 after BMT. Because CTX administered at days −7 and −6 is expected to primarily affect the recipient, we screened a wide array of recipient alterations pre-BMT in addition to recipient and donor alterations immediately after BMT. These included regulatory networks on which we have previously reported following TLI/ATS.17 To minimize selection bias, FLOCK algorithm25,26 statistical analysis was applied to PBMC samples obtained at day 0 pre-BMT and day 7 post-BMT from β-thal+/− recipients of TLI/ATS or TLI/ATS/CTX. FLOCK analysis demonstrated a significant increase in H-2Kd-negCD11b+Gr-1highCD11cneg cells (recipient MDSC) (P < .05) at day 0 pre-BMT (data not shown) and day 7 post-BMT (Figure 4A), and in H-2Kd+CD4+CD25+Foxp3+ cells (donor Treg) (P < .05) at day 7 post-BMT (Figure 4B) in β-thal+/− recipients of TLI/ATS/CTX compared with TLI/ATS.
We repeated these analyses in multiple strain combinations including BALB/c → B6. The latter MHC mismatch, similar to BALB/c → HW-80, afforded ready access to key B6 congenic and transgenic lines for adoptive transfer studies. B6 recipients of TLI/ATS/CTX + BALB/c BMT survived 100 days with stable mixed donor-recipient chimerism and no GVHD (data not shown). Addition of CTX to TLI/ATS in the BALB/c → B6 model significantly increased the recovery of splenic recipient MDSCs (P < .001) and H-2Kd-negB220negCD11b+Gr-1lowCD11c+ recipient MDCs (P < .001) at day 7 post-BMT (Figure 4C). The immunophenotype of recipient MDSCs and MDCs closely paralleled those reported following B6 BMT into TLI/ATS-conditioned BALB/c recipients,17 including surface expression of Ly6C, PD-1, PD-L1, PD-L2, CD1d, and CD124 (IL-4Rα) (supplemental Figure 1 on the Blood Web site). MDSCs from TLI/ATS/CTX- or TLI/ATS-conditioned recipients in the BALB/c → B6 model had surface marker expression previously reported in the B6 → BALB/c TLI/ATS model17 (supplemental Figure 2).
TLI/ATS/CTX-conditioned recipient MDSCs are necessary and sufficient to protect against lethal GVHD after MHC-mismatched BMT
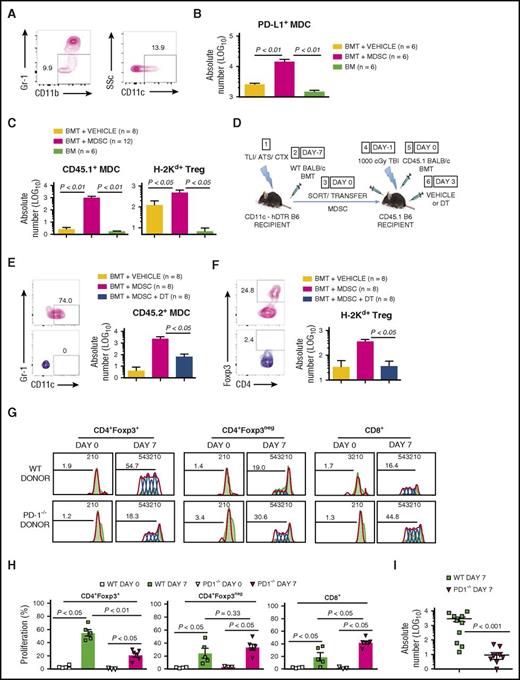
The Gr-1–depletive antibody clone RB6-8C5 or control isotype rat IgG2a17 was administered during TLI/ATS/CTX (Figure 4D). At day 0 pre-BMT (data not shown) and day 7 post-BMT, RB6-8C5 treatment significantly reduced recipient MDSC (P < .01), recipient MDC (P < .01), and donor Treg recovery (P < .01) among splenocytes (Figure 4E) and colonic intraepithelial lymphocytes (IELs) (data not shown), and resulted in significant GVHD compared with isotype control treatment (P < .01) (Figure 4F).
MDSCs sorted from spleens of TLI/ATS/CTX-conditioned CD45.1 B6 recipients of WT BALB/c BMT were transferred into WT (CD45.2) B6 recipients of myeloablative TBI (1000 cGy), 2 hours before secondary WT BALB/c BMT (106 BM cells and 106 SPL) (Figure 5A). The secondary BMT was designed to induce a subacute lethal experimental GVHD of similar chronicity and severity to that seen in the BALB/c → β−thal+/− HW-80 model. At day 7 post-BMT, adoptive recipients of 1.0 × 106 or 2.0 × 106 MDSCs demonstrated an MDSC dose-dependent reduction in donor CD8+ T-cell accumulation in colonic IEL compared with PBS vehicle controls (P < .05 and P < .01, respectively) (Figure 5B). Recipients of 2 × 106 MDSCs demonstrated a significant reduction in day 7 donor CD8+ T-cell accumulation in spleen (P < .01), MLN(P < .01), and colon (P < .001) (Figure 5C) and had significantly improved survival (P = .013) (Figure 5D), mean body mass (P = .001) (Figure 5E), cumulative GVHD scores (P < .001) (Figure 5F), and colonic GVHD scores (P < .001) (Figure 5F) compared with PBS-treated controls.
Adoptively transferred MDSCs generate recipient PD-L1+ MDCs and enhance donor CD4+Foxp3+ Treg recovery
By gating of CD45.1+ at day 7 (Figure 6A), we confirmed recovery of CD45.1+ PD-L1–expressing MDCs in spleens of adoptive recipients of MDSCs (P < .01) compared with vehicle control (Figure 6B). In day 7 recipient PBMC, there was significantly enhanced recovery of MDCs (P < .01) (Figure 6C) that express PD-L1 and PD-L2 (data not shown).17 We also confirmed increased recovery of donor Treg (P < .05) at day 7 in recipients of MDSCs compared with PBS controls (Figure 6C). Recovered Treg at day 7 were >95% Helios+ naturally occurring Treg (nTreg) (data not shown), as previously characterized17 ; these nTregs likely derive from peripheral T cells within the graft, because their recovery was significantly reduced (P < .05) in myeloablated B6 recipients of 50 × 106 BALB/c BM alone (Figure 6C, right).
Adoptively transferred GVHD-regulatory MDSCs enhance donor nTreg recovery through generation of PD-L1–expressing MDCs. (A) Representative FACS plots of peripheral blood in a WT B6 adoptive recipient at day 7 following infusion of 2 × 106 sorted CD45.1+ MDSCs and WT BALB/c BMT, as shown in Figure 5A. Left panel, gated live CD45.1+ cells demonstrating gating of CD11b+Gr-1low cells; right panel, gated live CD45.1+CD11b+Gr-1low cells demonstrating gating of side scatter (SSc) and CD11c to determine percentage MDCs. (B) Mean ± SEM absolute number (log 10) CD45.1+ recipient MDSC-derived MDCs expressing PD-L1 (PD-L1+ MDC) at day 7 post-BMT in spleens of adoptive recipients in treatment groups shown in Figure 5B. (C) Mean ± SEM absolute number (log 10) recipient MDSC-derived MDCs (CD45.1+ MDC) (left) and H-2Kd+CD4+CD25+Foxp3+ donor-derived Treg (H-2Kd+ Treg) (right) per 106 PBMC at day 7 following secondary BMT in adoptive recipients shown in Figure 5B. Data are cumulative (n = 5 experiments). (D) Protocol for depletion of MDSC-derived MDCs following MDSC adoptive transfer: (1) CD11c-hDTR transgenic (CD45.2) B6 mice received TLI/ATS/CTX followed by (2) CD45.1 BALB/c BMT. (3) At day 7 following BMT, H-2Kd-negB220negCD11b+Gr-1highCD11cneg MDSCs were sorted from spleens of recipients in (2) and 2 × 106 MDSCs infused IV (3) into adoptive CD45.1 B6 recipients of 1000 cGy TBI (4), followed by (5) CD45.1 BALB/c BMT. (5) Adoptive recipients of CD11c-hDTR transgenic MDSCs received either PBS vehicle control or 8 ng/g DT at 72 hours (day 3) post-BMT (6). Adoptive hosts were followed for 80 days for survival and signs of GVHD, with peripheral blood collected on days 7 and 28 to assess key immune subsets by FACS. BMT at day −7 = 50 × 106 bone marrow cells + 60 × 106 spleen cells from BALB/c donors; BMT at day 0 = 10 × 106 bone marrow cells + 10 × 106 spleen cells from BALB/c donors. (E) Representative FACS plots (left) and mean ± SEM absolute number (log 10) (right) CD45.2+ MDSC-derived MDCs (CD45.2+ MDC) per 106 PBMC in adoptive recipients shown in Figure 6D. Data represent n = 2 experiments. (F) Representative FACS plots (left) and mean ± SEM absolute number (log 10) H-2Kd+CD4+CD25+Foxp3+ donor-derived Treg (H-2Kd+ Treg) (right) in adoptive recipients shown in Figure 6D. Data represent n = 2 experiments. (G) Representative proliferation plots for CD4+Foxp3+ donor Treg, CD4+Foxp3neg donor CD4 effector, and CD8+ effector T cells among gated H-2Kd+TCRβ+ cells at day 7 in spleens of WT recipients of TLI/ATS/CTX conditioning and BMT from WT (top) or PD-1−/− donors (bottom). Data represent a total of n = 10-15 individual mice over n = 2 separate experiments. (H) Mean ± SEM proliferation for CD4+Foxp3+ donor Treg, CD4+Foxp3neg donor CD4 effector, and CD8+ effector T cells among gated H-2Kd+TCRβ+ cells at day 7 in spleens of WT recipients of TLI/ATS/CTX conditioning and BMT from WT (top) or PD-1−/− donors (bottom). Data represent pooled samples from 2 to 3 animals per data point (n = 10 WT and n = 15 PD-1−/−) over n = 2 experiments. (I) Mean ± SEM absolute number (log 10) CD4+Foxp3+ donor Treg among colonic IELs at day 7 in mice represented in panel H. Data represent a total of n = 10-15 individual mice per group over n = 2 separate experiments.
Adoptively transferred GVHD-regulatory MDSCs enhance donor nTreg recovery through generation of PD-L1–expressing MDCs. (A) Representative FACS plots of peripheral blood in a WT B6 adoptive recipient at day 7 following infusion of 2 × 106 sorted CD45.1+ MDSCs and WT BALB/c BMT, as shown in Figure 5A. Left panel, gated live CD45.1+ cells demonstrating gating of CD11b+Gr-1low cells; right panel, gated live CD45.1+CD11b+Gr-1low cells demonstrating gating of side scatter (SSc) and CD11c to determine percentage MDCs. (B) Mean ± SEM absolute number (log 10) CD45.1+ recipient MDSC-derived MDCs expressing PD-L1 (PD-L1+ MDC) at day 7 post-BMT in spleens of adoptive recipients in treatment groups shown in Figure 5B. (C) Mean ± SEM absolute number (log 10) recipient MDSC-derived MDCs (CD45.1+ MDC) (left) and H-2Kd+CD4+CD25+Foxp3+ donor-derived Treg (H-2Kd+ Treg) (right) per 106 PBMC at day 7 following secondary BMT in adoptive recipients shown in Figure 5B. Data are cumulative (n = 5 experiments). (D) Protocol for depletion of MDSC-derived MDCs following MDSC adoptive transfer: (1) CD11c-hDTR transgenic (CD45.2) B6 mice received TLI/ATS/CTX followed by (2) CD45.1 BALB/c BMT. (3) At day 7 following BMT, H-2Kd-negB220negCD11b+Gr-1highCD11cneg MDSCs were sorted from spleens of recipients in (2) and 2 × 106 MDSCs infused IV (3) into adoptive CD45.1 B6 recipients of 1000 cGy TBI (4), followed by (5) CD45.1 BALB/c BMT. (5) Adoptive recipients of CD11c-hDTR transgenic MDSCs received either PBS vehicle control or 8 ng/g DT at 72 hours (day 3) post-BMT (6). Adoptive hosts were followed for 80 days for survival and signs of GVHD, with peripheral blood collected on days 7 and 28 to assess key immune subsets by FACS. BMT at day −7 = 50 × 106 bone marrow cells + 60 × 106 spleen cells from BALB/c donors; BMT at day 0 = 10 × 106 bone marrow cells + 10 × 106 spleen cells from BALB/c donors. (E) Representative FACS plots (left) and mean ± SEM absolute number (log 10) (right) CD45.2+ MDSC-derived MDCs (CD45.2+ MDC) per 106 PBMC in adoptive recipients shown in Figure 6D. Data represent n = 2 experiments. (F) Representative FACS plots (left) and mean ± SEM absolute number (log 10) H-2Kd+CD4+CD25+Foxp3+ donor-derived Treg (H-2Kd+ Treg) (right) in adoptive recipients shown in Figure 6D. Data represent n = 2 experiments. (G) Representative proliferation plots for CD4+Foxp3+ donor Treg, CD4+Foxp3neg donor CD4 effector, and CD8+ effector T cells among gated H-2Kd+TCRβ+ cells at day 7 in spleens of WT recipients of TLI/ATS/CTX conditioning and BMT from WT (top) or PD-1−/− donors (bottom). Data represent a total of n = 10-15 individual mice over n = 2 separate experiments. (H) Mean ± SEM proliferation for CD4+Foxp3+ donor Treg, CD4+Foxp3neg donor CD4 effector, and CD8+ effector T cells among gated H-2Kd+TCRβ+ cells at day 7 in spleens of WT recipients of TLI/ATS/CTX conditioning and BMT from WT (top) or PD-1−/− donors (bottom). Data represent pooled samples from 2 to 3 animals per data point (n = 10 WT and n = 15 PD-1−/−) over n = 2 experiments. (I) Mean ± SEM absolute number (log 10) CD4+Foxp3+ donor Treg among colonic IELs at day 7 in mice represented in panel H. Data represent a total of n = 10-15 individual mice per group over n = 2 separate experiments.
CD11c-hDTR MDSC adoptive transfer followed by DT treatment abrogates MDC generation and reduces donor nTreg recovery
To investigate whether augmented donor nTreg recovery after MDSC adoptive transfers required recipient MDC generation from MDSCs, we transferred MDSCs sorted from TLI/ATS/CTX-conditioned B6 mice expressing hDTR under the control of the CD11c promoter (B6 CD11c-hDTR) into CD45.1+ B6 adoptive recipients, treating adoptive recipients with diphtheria toxin (DT) or PBS vehicle 72 hours post-BMT (Figure 6D). As compared with MDSC + (PBS) vehicle, MDSC + DT treatment resulted in significant reduction of CD45.2+ MDC recovery in PBMC at day 7 (data not shown) and day 28 (P < .05) (Figure 6E), concomitant with a significant decrease in donor Treg recovery (P < .05) (Figure 6F). Although a trend toward poorer survival was seen in DT-treated MDSC recipients (data not shown), long-term GVHD survival differences could not be assessed because of recovery of CD45.2+ DCs after 40 days with currently available DT administration protocols. We are currently optimizing a DT administration protocol to allow long-term CD11c+ cell depletion without inordinate DT toxicity.
BMT from PD-1 deficient compared with WT donors results in reduced proliferation and recovery of donor Treg after TLI/ATS/CTX and MHC-mismatched BMT
To investigate the requirement for PD-1/PD-ligand signaling in the generation of donor nTreg and the control of donor CD8 effector T-cell proliferation early after TLI/ATS/CTX conditioning, BMT including splenocytes prelabeled with e450 proliferation dye was performed from PD-1−/− vs WT donors to TLI/ATS/CTX-conditioned WT recipients. At day 7 after BMT, harvest and analysis of key GVHD target organs confirmed significantly reduced splenic donor Foxp3+ nTreg (P < .01), enhanced splenic donor CD8 effector T-cell proliferation (P < .05) (Figure 6G-J), and significantly reduced donor nTreg recovery in key target organs, including colon (P < .001) (Figure 6I), following PD-1−/− donor BMT. Of note, gated donor splenic TCRβ+CD4+Foxp3neg effector T-cell proliferation was not significantly different between recipients of PD-1−/− and WT donor BMT (P = .33) (Figure 6G-H).
Discussion
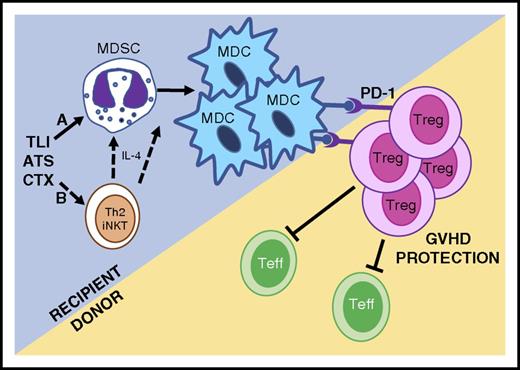
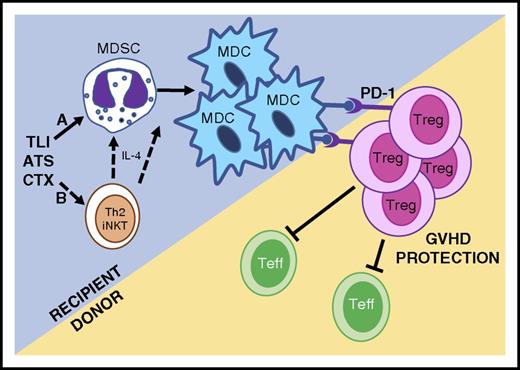
Because anti-Gr-1 antibody treatment during TLI/ATS/CTX depleted both MDSCs and MDCs and abrogated GVH tolerance (Figure 4), we hypothesized that Gr-1highCD11cneg MDSCs spared by this nonmyeloablative conditioning could be the source of regulatory Gr-1lowCD11c+ MDCs.17 Using adoptive transfers of congenically marked and CD11c-hDTR transgenic recipient-type MDSCs (Figure 5), we ascertained that TLI/ATS/CTX-derived MDSCs can convert to PD-ligand–expressing MDCs, and that this conversion is associated with enhanced recovery of donor-type Treg and protection from GVHD. The PD-1/PD-1 ligand axis dependence of this recipient-donor loop was confirmed by finding reduced in vivo proliferation and reduced nTreg recovery early after TLI/ATS/CTX and BMT from PD-1–deficient donors (Figure 6G-I). These in vivo mechanistic data confirm our prior in vitro finding that induction of donor Treg proliferation by recipient myeloid regulatory cells is PD-1 signaling dependent.17 The associated finding that donor CD8 effector T-cell proliferation is enhanced in PD-1−/− donor transfers also supports our prior published result that Tregs regulate CD8-mediated colitis after TLI-based conditioning and BMT.15 Furthermore, the finding that CD4 effector T-cell proliferation is not enhanced in PD-1−/− compared with WT donor BMT supports the notion that the donor Treg proliferation is not nonspecific (lymphopenia-driven or a pan-CD4 T-cell dysfunction-mediated). A mechanistic summary of confirmed and postulated components of the innate-adaptive immune tolerance axis after TLI/ATS/CTX nonmyeloablative conditioning is provided in Figure 7.
Proposed mechanisms of allogeneic tolerance induction after TLI/ATS/CTX-based conditioning and MHC-mismatched BMT. (Mechanism A) TLI/ATS/CTX conditioning is nonmyeloablative, allowing the maintenance and expansion of key recipient myeloid regulatory subsets, including MDSCs. MDSCs convert to regulatory PD-ligand expressing MDCs in the context of MHC-mismatched BMT, facilitating PD-1–dependent in vivo expansion of donor Treg. Donor Treg regulate donor Teffector (Teff) cell expansion and GVHD. (Mechanism B) Based upon robust data in TLI/ATS models,15,17 we postulate that TLI/ATS/CTX may facilitate Th2 polarized iNKT-derived IL-4 secretion, which further drives recipient MDSC generation and/or conversion of MDSC to MDC in the setting of MHC-mismatched BMT. Mechanisms in B are currently being investigated using iNKT-deficient and STAT6-deficient models.
Proposed mechanisms of allogeneic tolerance induction after TLI/ATS/CTX-based conditioning and MHC-mismatched BMT. (Mechanism A) TLI/ATS/CTX conditioning is nonmyeloablative, allowing the maintenance and expansion of key recipient myeloid regulatory subsets, including MDSCs. MDSCs convert to regulatory PD-ligand expressing MDCs in the context of MHC-mismatched BMT, facilitating PD-1–dependent in vivo expansion of donor Treg. Donor Treg regulate donor Teffector (Teff) cell expansion and GVHD. (Mechanism B) Based upon robust data in TLI/ATS models,15,17 we postulate that TLI/ATS/CTX may facilitate Th2 polarized iNKT-derived IL-4 secretion, which further drives recipient MDSC generation and/or conversion of MDSC to MDC in the setting of MHC-mismatched BMT. Mechanisms in B are currently being investigated using iNKT-deficient and STAT6-deficient models.
MDSCs can suppress both allogeneic and syngeneic effector T-cell responses by multiple mechanisms,20-22 including STAT6-dependent expression of arginase 1.20,28,29 Ex vivo expanded donor-type MDSCs generated with IL-13 have been shown to regulate B6 → BALB/c experimental GVHD following lethal TBI conditioning.22 However, the possibility that either recipient or donor MDSCs convert to regulatory DCs or alter nTreg generation has not, to our knowledge, been investigated. We propose that direct T-cell modulation by MDSCs22 is not the only mechanism these cells use in regulating GVHD. In the CD11c-hDTR-transgenic MDSC transfers, we confirmed that MDSC→MDC conversion does occur, and we attempted to define whether long-term GVHD regulation by MDSCs required this conversion to MDCs. The latter studies were impeded by suboptimal long-range DC depletion or inordinate off-target toxicity with existing DT administration protocols, limitations we are currently addressing. We are currently defining the role of Th2 polarization and iNKT cells in development of MDSCs and/or MDSC conversion to MDC after TLI/ATS/CTX (Figure 7B). We are also examining whether these MDSCs can directly regulate MHC-mismatched CD8 effector responses, and whether MDSCs or MDCs play a role in engraftment facilitation (HVG immune tolerance). These studies are expected to provide further insights into innate immune mechanisms by which nonmyeloablative conditioning strategies can augment bidirectional immune tolerance.
CTX has been well-studied in both marrow and organ graft tolerance in WT mice.30-33 For example, a nonmyeloablative regimen of focal thymic irradiation, anti-CD4 and anti-CD8 antibodies, and CTX (200 mg/kg) induces durable immune tolerance in WT recipients of MHC-mismatched BMT.31 Regulatory T cell–dependent mechanisms have also been postulated for CTX-associated HVG tolerance to MHC-mismatched skin allografts.33 GVHD protection with intact GVH reactivity has been reported in CTX-based regimens.34,35 Carefully timed administration of systemic CTX has been exploited in the nonmyeloablative setting to selectively deplete allo-reactive donor anti-recipient T cells36 and/or to deplete recipient anti-donor effector T cells in WT recipients.37,38 Recently, a central role for donor Treg expansion has also been reported in CTX tolerance induction regimens.39 However, to date, no studies have evaluated recipient MDSC or MDC generation after CTX. Furthermore, no reports have evaluated TLI/ATS or CTX-containing conditioning for tolerance induction in hemoglobinopathies.
The current studies in β-thal+/− mice demonstrate disease correction with improved engraftment, GVHD protection, and overall survival following TLI/ATS/CTX conditioning and MHC-mismatched BMT compared with outcomes after TLI/ATS or TBI/ATS/CTX. A key advantage of this β-thal+/− preclinical model system is the relatively increased (disease-associated) resistance to MHC-mismatched donor cell engraftment when β-thal+/− HW-80 mice receive the same conditioning (TLI/ATS) as their WT counterparts (Figure 1B). Ongoing model development is aimed at mimicking the well-documented clinical engraftment barrier of donor-specific antibodies seen in chronically transfused hemoglobinopathy patients.10-12 We are achieving this by pretransfusing recipient mice with autologous control, donor type, or third-party blood and measuring anti-donor antibody development before initiation of conditioning. These models will be tested with TLI-based conditioning strategies to determine whether allo-antibody–induced engraftment barriers can be overcome.
TLI/ATS/CTX recipients exhibited long-term mixed donor-recipient hematopoiesis (stable mixed chimerism), a well-documented feature of immune tolerance in murine models15,17,30-34 that is much less frequently achieved in humans. Notably, alemtuzumab-containing conditioning and posttransplant sirolimus immunoprophylaxis has achieved stable mixed chimerism in humans after HLA-matched HCT for sickle hemoglobinopathies.40 We propose that the innate-adaptive immune regulatory milieu of TLI/ATG combined with CTX or other alkylator conditioning pre-BMT and alkylator and/or sirolimus immunoprophylaxis post-BMT41 should facilitate potent regulatory immune tolerance following both HLA-matched and HLA-mismatched HCT for hemoglobinopathies. We are investigating whether other alkylators (melphalan, thiotepa) in combination with TLI/ATS can augment MHC-mismatched transplant tolerance in murine models of β-thal and sickle cell disease.42
These studies provide mechanistic evidence that recipient MDSCs can develop into PD-ligand expressing MDCs, augment donor Treg recovery, and regulate GVHD after BMT, thereby delineating novel recipient innate regulatory mechanisms relevant to MHC-mismatched HCT. These preclinical data also provide fundamental mechanistic insight into allogeneic immune tolerance associated with CTX and possibly with other alkylators, thus defining means to augment bidirectional immune tolerance in TLI/ATG regimens in humans. These findings should facilitate development of novel reduced toxicity conditioning in MHC-mismatched HCT for thalassemia and sickle cell disorders, nonmalignant disorders with significant propensity for graft rejection and GVHD.
Presented in abstract form at the 2015 Tandem Meetings of American Society for Blood and Marrow Transplantation/Center for International Blood and Marrow Transplant Research, San Diego, CA, 14 February 2015.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank J. Houston, L. He, S. Perry, and R. Ashmun for cell sorting; St. Jude and University of Miami veterinary staff for animal husbandry; H. Abdelsamed for technical contributions and figure preparation; K. Andrews and M. Yoshida for technical support; and R. Levy (University of Miami) and N. Chao (Duke University) for manuscript critiques.
This work was supported by the Lemuel Diggs Endowed Fellowship in Sickle Cell Disease at St. Jude (H. Abdelsamed), V Foundation for Cancer Research Scholar Award (A.B.P.), National Institutes of Health, National Heart, Lung, and Blood Institute (1K08HL088260 and 1R56HL125449) (A.B.P.), the American Lebanese Syrian Association Charities (S.E., A.S., P.V., T.O., A.B.P.), and the Batchelor Foundation (S.E., A.B.P.).
Authorship
Contribution: S.E., A.S., M.S., and T.O. performed experiments and analyzed results; P.V. analyzed results and made the figures; and A.B.P. conceived and designed research, performed experiments, analyzed results, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Asha B. Pillai, Division of Pediatric Hematology/Oncology/Stem Cell Transplantation, Departments of Pediatrics and Microbiology and Immunology, Rm 745, Batchelor Children’s Research Institute, 1580 NW 10th Ave, Miami, FL 33136; e-mail: asha.pillai@med.miami.edu.
References
Author notes
S.E. and A.S. contributed equally to this study.