Abstract
Hemoglobin (Hb) Bart's hydrops fetalis syndrome (BHFS) resulting from α0-thalassemia is considered a universally fatal disorder. However, over the last 3 decades, improvements in intrauterine interventions and perinatal intensive care have resulted in increasing numbers of BHFS survivors. We have initiated an international registry containing information on 69 patients, of which 31 are previously unpublished. In this perspective, we analyze the available clinical information to document the natural history of BHFS. In the future, once we have accrued sufficient cases, we aim to build on this study and provide information to allow counseling of at-risk couples. To date, 39 patients have survived beyond the age of 5 years, 18 of whom are now older than 10 years. Based on the available cases, we find evidence to suggest that intrauterine therapy provides benefits during the perinatal and neonatal period; however, it may not provide additional benefits to long-term growth and neurodevelopmental outcomes. Growth retardation is a major adverse long-term outcome among BHFS patients with ∼40% being severely affected in terms of weight and ∼50% in terms of height. There is also an increased risk of neurodevelopmental delay as we find 20% (11/55) of BHFS survivors suffer from a serious delay of ≥6 months. Most patients in the registry require lifelong transfusion and often have associated congenital abnormalities and comorbidities. This perspective is a first step in gathering information to allow provision of informed counseling on the predicted outcomes of affected babies.
Introduction
α-Thalassemia, arising from the underproduction or absence of α-globin synthesis, is one of the most common human monogenic disorders with a carrier rate of >1% among all tropical and subtropical populations studied.1 Reduction of α-globin results in aggregations of excess β-like globin, which during fetal life consists of γ-globin (γ4), termed Hemoglobin (Hb) Bart's; postnatally, β-globin forms tetramers (β4) termed HbH (Table 1). Both Hb Bart's and HbH have increased oxygen affinity resulting in poor oxygen delivery. Excessive β-like globin chains also damage maturing erythroid precursors, causing ineffective erythropoiesis. Moreover, α-thalassemia predominantly leads to hemolysis, resulting from the premature destruction of mature red blood cells carrying Hb Bart's and HbH.2
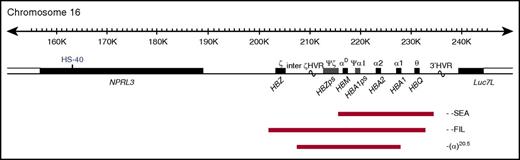
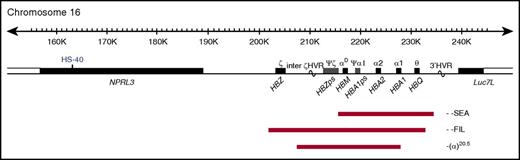
The α-globin locus contains 3 functional genes: HBZ, HBA2, and HBA1 (Figure 1); all 3 are expressed during primitive erythropoiesis. However, from 6 to 8 weeks of gestation, HBZ is repressed and remains quiescent throughout fetal and adult life.3 α-Thalassemia most commonly results from deletion of 1 (−α) or both (−−) α-globin genes. Carriers of α-thalassemia (−α/αα and −−/αα) and homozygotes for the mild haplotype −α (−α/−α) have mild hypochromic microcytic anemia. Compound heterozygotes (−−/−α) have hemolytic anemia (HbH disease), whereas homozygotes for the −− haplotype have a lethal condition known as Hb Bart's hydrops fetalis syndrome (BHFS). To date, ∼40 α0-thalssemia deletions that remove both HBA1 and HBA2 have been reported4,5; the most common is the Southeast Asian (SEA) deletion, a 20.5-kb deletion that removes both HBA2 and HBA1, leaving HBZ intact (Figure 1).6,7
Map of α-globin deletions found in the BHFS survivors. The α-globin cluster on human chromosome 16 (16p13.3). Genes in the region and the major α-cis-regulatory element (HS-40) are shown in the middle panel. The extent of the deletions found in the BHFS survivors reported in this study is shown by red bars. HVR, hypervariable region; ps, pseudogene. (Locations refer to reference genome hg19.)
Map of α-globin deletions found in the BHFS survivors. The α-globin cluster on human chromosome 16 (16p13.3). Genes in the region and the major α-cis-regulatory element (HS-40) are shown in the middle panel. The extent of the deletions found in the BHFS survivors reported in this study is shown by red bars. HVR, hypervariable region; ps, pseudogene. (Locations refer to reference genome hg19.)
BHFS fetuses suffer from severe anemia in utero, causing severe tissue hypoxia, heart failure and a range of developmental abnormalities associated with hydrops fetalis.8 Individuals with at least one copy of HBZ produce a small amount of functional embryonic Hb Portland I (ζ2γ2) until the gene is silenced, allowing survival to the third trimester of pregnancy.
BHFS has also been described in individuals with compound heterozygosity for the −−SEA and 2 less common α0-thalassemia deletions, −−FIL and −−THAI, that span the ζ- and α-globin genes.9 Rare cases of BHFS have been reported in individuals of Greek10-13 and Sardinian origin.14
The SEA deletion is present at an allele frequency of 4% to 8% in Southern China and Hong Kong15-17; it is also found at high frequency in Thai, Filipino, and Vietnamese populations.18 However, it is most common in Northern Thailand (allele frequency up to ∼14%).19,20 BHFS is therefore a major global health problem with at least 26 000 at-risk pregnancies and ∼6600 affected fetuses in these regions annually. Screening followed by prenatal prevention of homozygous α0-thalassemia births is routine in areas with a high carrier rate,21-23 and this strategy represents a critical safeguard to public health. Nevertheless, improvements in intrauterine intervention and postnatal intensive care have resulted in increasing reports of BHFS survivors; however, the impact of this disease on neurological and physical development is unclear. In 2013, we initiated a registry of BHFS patients, which currently contains clinical information from 69 individuals including 31 previously unreported cases. The major aim of this on-going registry is to gather information to allow informed counseling of rare parents who may wish to consider continuing BHFS pregnancies. This retrospective study is a first step toward our ultimate aim of providing a resource for clinicians managing BHFS pregnancies and patients. Ethical approval for the cases presented was provided by the Oxfordshire Research Ethics Committee (reference: MREC 03/8/097) to D.R.H.
General characteristics of BHFS survivors
Among the group of 38 previously reported patients, clinical information from 15 has been updated.24-58 The launch of this registry has brought to light a further 31 unpublished BHFS patients. The patients’ ages at the time of report or last communication are shown in Table 2; the oldest is currently 31 years old. Table 2 shows 59% (41/69) of patients underwent at least 1 episode of intrauterine intervention. Most of the remaining 28 patients who survived naturally until birth received transfusion within the first day postpartum. Geographical distribution of patients is shown in Table 3. α-Globin genotypes are reported in 67 patients: 50 of whom are homozygous for the SEA deletion (−−SEA/−−SEA); 2 have compound heterozygosity for the SEA and the Filipino deletions (−−SEA/−−FIL); 1 is homozygous for the −(α)20.5 deletion; and 14 patients are reported to have deletion of all 4 α-globin genes. Diagnosis of BHFS was made in the 2 remaining patients by Hb electrophoresis showing Hb Bart's to be the major globin present.
Prenatal diagnosis and intrauterine management
All 4 patients born before 1990 survived naturally until birth without intrauterine treatment, whereas 17 of 29 patients (59%) born in the 1990s, 13 of 24 (54%) born in the 2000s, and all 11 patients born after 2010 (3 patients from the United States, 3 from Canada, 2 from Hong Kong, and 1 each from Turkey, Sweden, and Poland) received either intrauterine transfusions or hematopoietic stem cell (HSC) infusions. Prenatal diagnosis of BHFS was made at a median gestational age (GA) of 22 weeks (range 10-31 weeks) most frequently from ultrasonographic findings of hydrops fetalis followed by fetal DNA analysis and cord blood Hb electrophoresis revealing Hb Bart's (γ4) to be the most abundant form of Hb. Of 41 patients treated in utero, 80% (33/41) received red cell transfusion alone; 15% (6/41) underwent exchange transfusion, and 5% (2/41) underwent HSC transplantation. The earliest GA at which intrauterine intervention was performed was 13 weeks.34
Of the affected fetuses who received intrauterine treatment, 59% (24/41) received their initial blood transfusion via the umbilical vein at GA 20-29 weeks (median 26 weeks, range 13-32 weeks; Table 2). Intrauterine exchange transfusions were performed in 6 patients,29,33,43,51 3 of whom had reported fetal ascites as the predominant sign.29,33,43 Other reasons reported for favoring exchange transfusion were removal of nonfunctional Hb Bart's (γ4) and avoidance of fetal volume overload that may aggravate existing poor cardiac function.
Intrauterine HSC transplantation was performed in 2 patients participating in research studies. Fetal liver cells derived from legally aborted fetuses were used to source HSCs for 2 in utero infusions in 1 patient,30 and haploidentical paternal CD34+ cells were used for 3 stem cell infusions in the other.34 HSC infusion was initiated early in the second trimester of pregnancy (GA 15 and 13 weeks). Although 1 patient received additional HSC infusion at 3 months of age,34 both patients remain transfusion dependent, consistent with unsuccessful engraftment.
Intrauterine intervention appeared to prolong the course of pregnancies as the frequency of term delivery increased from 3 of 26 pregnancies (12%) without treatment to 16 of 40 pregnancies (40%) when interventions were employed. The median GA at birth of affected fetuses treated in utero is 36 weeks; this is significantly higher than those surviving naturally until birth (median GA at birth 32 weeks, P = .0002). Despite these apparent benefits, intrauterine intervention resulted in intrauterine infection and termination of pregnancy in 2 cases.33,35
Maternal complications
Current data, in keeping with previous reports,59,60 suggest an increased incidence of serious maternal complications in BHFS pregnancies. Preterm delivery is the most common obstetric complication, occurring in 47 of 66 pregnancies (71%). In addition, in the antenatal period, the majority of problems arise in the third trimester with the median GA at detection being 29 weeks (range 22-34 weeks). Complications include polyhydramnios, oligohydramnios, intrauterine infection, preeclampsia and abruptio placenta (summarized in Table 4). The frequency of antepartum and postpartum maternal complications is not significantly different between the in utero treated group (11/25, 44%) and the untreated group (8/16, 50%, P = .71).
Neonatal course of BHFS survivors
Clinical presentation of BHFS newborns includes anemia, hydrops, hepatosplenomegaly, and respiratory distress. In 54 of 69 patients (78%), BHFS was detected in utero, and in the remainder, BHFS was diagnosed by Hb electrophoresis, showing Hb Bart's (γ4) to be the major Hb at birth. The presence of Hb Portland I (ζ2γ2) at birth (median 17%, range 5.2% to 25.4%), indicating persistent expression of the ζ-globin gene, was reported in at least 14 patients in the untreated group. Causes of hydrops remain unclear8,61; however, intrauterine intervention was associated with a lower incidence of hydrops (4/24 patients, 17%) compared with untreated cases (12/22 patients, 55%) (Table 5).
There were significantly higher median Apgar scores at 1 and 5 minutes after birth in patients treated in utero (Table 5). This difference suggests that intrauterine treatment of BHFS also leads to a lower likelihood of fetal distress and a better response to initial resuscitation and thus less chance of severe birth asphyxia. Supporting this, a significantly longer duration of neonatal ventilation was required in those untreated in utero (Table 5).
Other serious neonatal complications reported, irrespective of intrauterine treatment, include persistent pulmonary hypertension, respiratory distress syndrome requiring surfactant therapy, pulmonary hemorrhage, convulsions, cortical brain infarction, mild intraventricular hemorrhage, bilateral pneumothorax, pericardial effusion, septicemia, severe thrombocytopenia, and portal vein thrombosis. In addition, abdominal and pleural paracentesis were required immediately after birth in 2 hydropic infants. These data suggest that BHFS infants often experience a difficult neonatal period and intrauterine treatment is often associated with a less stormy neonatal course (summarized in Table 5).
Congenital abnormalities
A high frequency of congenital abnormalities is observed in patients: overall 37 of the 58 patients (64%), where data are available, had at least 1 anomaly (Table 6). This incidence does not differ between in utero treated (22/36 patients, 61%) and untreated patients (15/22, 68%). The most common malformations are urogenital abnormalities, which occur almost exclusively in male patients, with hypospadias being the most frequent (present in 57% [21/37] of male patients). Other abnormalities include undescended testes, ambiguous genitalia, bifid scrotum, micropenis, hydrocele, and hydronephrosis. Although correctable by surgery, these abnormalities often remain as an important source of distress and reduced quality of life.
Limb abnormalities are also fairly common, occurring in 9/58 patients (16%; Table 6) and range from relatively mild deformities, such as asymmetrical hand size, to major abnormalities such as complete absence of the distal elements of the foot. Limb malformations remain lifelong; however, in many cases, they are mild and may not significantly impact patients.
Long-term growth outcomes
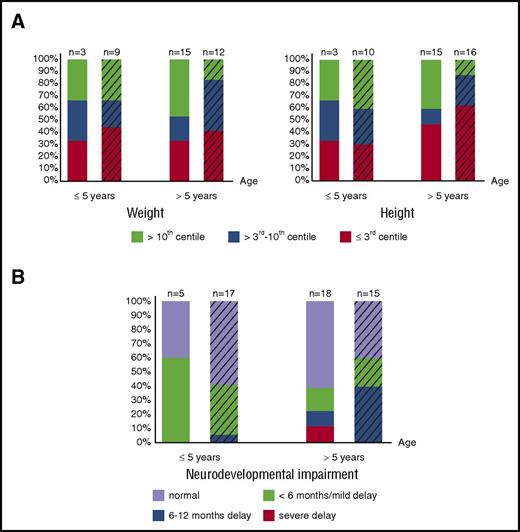
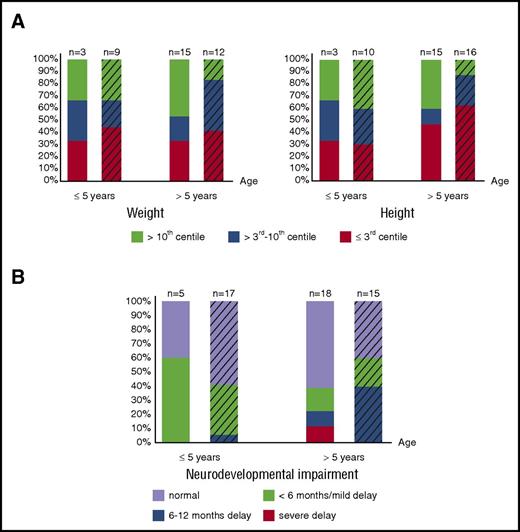
Growth data (assessed by weight and height of matched populations) are available in 18 of the 28 patients who survived naturally until birth and in 26 of 41 patients transfused in utero. Although the proportion of patients suffering from severe growth retardation varies between age groups, 31% (12/39) are severely affected by weight and height; an additional 8% (3/39) have severely affected weight, and an additional 20% (9/44) have severely affected height (weight/height ≤3rd centile). Therefore, impaired growth remains an important, long-term, adverse outcome affecting a large number of patients, regardless of intrauterine treatment and neonatal course (Figure 2A), suggesting abnormalities in long-term growth of patients with BHFS, similar to β-thalassemia major,63 are multifactorial.
Long-term growth and neurodevelopmental impairment of BHFS survivors. (A) Growth assessed by weight (left panel) and height (right panel) and degree of neurodevelopmental impairment (B) of patients who received intrauterine treatment (striped bars) and who survived naturally until birth are shown. The x-axis indicates age at the time of last assessment. The y-axis shows the proportion of the BHFS survivors. Color codes below the graphs indicate weight, height, and neurodevelopmental impairment levels as described. Numbers of patients with available reports are shown on the top of each bar.
Long-term growth and neurodevelopmental impairment of BHFS survivors. (A) Growth assessed by weight (left panel) and height (right panel) and degree of neurodevelopmental impairment (B) of patients who received intrauterine treatment (striped bars) and who survived naturally until birth are shown. The x-axis indicates age at the time of last assessment. The y-axis shows the proportion of the BHFS survivors. Color codes below the graphs indicate weight, height, and neurodevelopmental impairment levels as described. Numbers of patients with available reports are shown on the top of each bar.
Of note, patients who survived naturally until birth and who have long-term weight and height of >3rd centile (n = 10), in any given age group, were born more prematurely (median GA at birth 29 weeks) than those with severe growth retardation (n = 8, median GA at birth 32 weeks, P = .04). These data suggest that premature delivery, allowing intensive postnatal intervention and life support including blood transfusion, may contribute to better growth outcomes.
Postnatal HSC transplantation was successfully performed in 14/69 patients (20%), resulting in transfusion-independent individuals. However, poor growth, documented at least 2 years after transplantation (range 2-18 years), was corrected in only 4 of 10 patients in whom transplantation was performed early in life (<5 years of age). The remaining cases in this group remain growth retarded, weight and height being similarly affected, suggesting improved growth does not necessarily result from curative therapy. Frequency of blood transfusion given to non–transplanted patients varied. An institution in Thailand, which is treating 4 transfusion-dependent survivors, reported significant improvement of weight and height during the first year following initiation of hypertransfusion. These patients were transfused to maintain pretransfusional “functional” Hb (HbA + HbF, but not HbH) levels of 10 g/dL for at least 6 years at the time of last report. Of the 4 patients treated in this way, 3 have long-term normal growth and 1 has continuously improving growth. The benefits of this strategy are supported by a recent report of 4 patients treated with a similar regimen for at least 1 year.57 Growth hormone (GH) deficiency was documented in 3 patients, all of whom responded to GH therapy. Therefore, improved transfusion regimes are likely to offer significant benefits to BHFS patients.
Neurodevelopmental outcomes
A previous review reported affected fetuses to have reduced brain weight relative to GA from ∼8 weeks onwards.2 Neurodevelopmental outcomes are documented in 23 of 28 patients who survived naturally until birth and in 32 of 41 patients maintained with intrauterine treatment. Most reports were made at age >5 years in both groups and as such represent “long-term” outcomes (Figure 2B). Assessment of neurodevelopmental delay was divided by severity into 4 categories according to evidence either from outpatient-based developmental milestone assessments (n = 10) and age-appropriate formal child developmental assessment (n = 12) or from rating by referring physicians (n = 33): (1) severe delay; (2) 6-12 months’ delay; this category also includes delay of 1 to 2 life skills and significant intellectual delay (+/−) requiring special education; (3) <6 months or “mild” delay; (4) normal development.
Of patients >5 years old, 14 of 18 patients (78%) in the non–intrauterine treatment group and 9 of 15 (60%) in the intrauterine treatment group have “normal” neurodevelopment or only “mild” delay (Figure 2B). Nevertheless, 4 patients untreated in utero and 7 patients treated in utero from all age groups suffer from significant neurodevelopmental delay of ≥6 months. These data suggest that, in our cohort, although most patients at any given age have favorable long-term neurodevelopmental outcomes (either “normal” or “mild” delay) irrespective of intrauterine treatment (Figure 2B), as many as 20% (11/55 patients) suffer unfavorable outcomes.
Seven of 9 patients, including both intrauterine-treated and untreated groups, with 6- to 12-months or severe developmental delay experienced a difficult neonatal period. Difficult neonatal events included neonatal resuscitation, requirement for mechanical ventilation for >3 days, intraventricular hemorrhage, and cortical infarction. However, 21 of 28 patients (75%) who experienced such neonatal events developed normally in the long term, implying that survivors with favorable neurodevelopmental outcomes may have a critical neonatal period; however, those with significant delay are more likely to have had a difficult neonatal course.
Significant neurodevelopmental delay remains in 3 of 10 patients transplanted at <5 years of age who are transfusion independent. Therefore, similar to growth, neurodevelopmental delay may not always be recoverable by early curative therapy. Three of the 4 patients hypertransfused for at least 6 years (current age 7 to 14 years) have normal neurodevelopment. The remaining patient (aged 6 years) has 6- to 12-months delay; however, this is improved from the severe delay reported prior to hypertransfusion. One recently reported patient57 received a similar regimen since birth and achieved normal developmental milestones at 14 months of age. Whether this recently implemented transfusion regimen consistently results in favorable long-term neurodevelopmental outcomes as well as growth remains to be seen.
Current treatments
Most patients require lifelong transfusion. Curative HSC transplantation is mainly restricted by availability of donors and limitations of funding, especially in less developed countries. In this study, 18 of 69 patients (26%) have undergone postnatal HSC transplantation, of which 14 have become transfusion independent (Table 2). In most of the cases that became transfusion independent (10/14; 71%), transplants were performed early in life, ranging from 12 to 44 months of age. Although this early curative therapy may not always result in correction of poor growth and developmental delay, these patients have a reduced risk of chronic transfusion-related complications, such as iron overload. Transplantation was performed using either HLA-matched or -mismatched cells obtained from bone marrow, cord blood, or peripheral blood of siblings or unrelated donors. Significant transplant-related complications, including acute skin and gut graft-versus-host disease, posttransplant lymphoproliferative disorder due to Epstein-Barr virus, and hearing loss resulting from chemotherapeutic agents used for the conditioning regimen, were noted in 3 patients. Of the 4 unsuccessfully treated cases, HSC transplantations resulted in lethality in 1 case58 and were unsuccessful because of graft rejection in 3 patients, who remain transfusion dependent.
A total of 53 of 69 patients (77%, Table 2) are transfusion dependent and the majority of these patients have been regularly transfused starting from a few days to a few weeks after birth. The frequency of transfusions varies from 2 to 5 weeks, and survivors are most often treated to maintain a pretransfusional Hb level range from 7 to 10 g/dL.
A recent study of 4 BHFS patients (aged 6-16 years) suggests that hemolytic anemia leading to extramedullary hematopoiesis is the most prominent pathophysiology in survivors rather than ineffective erythropoiesis.57 The most apparent manifestation of extramedullary hematopoiesis is the presence of splenomegaly and progressive levels of peripheral reticulocytes in these patients, as well as nonfunctional HbH(β4).57 A more intensive transfusion strategy based on the functional Hb level, calculated by total Hb × (1 − HbH/100), of >10 g/dL as a parameter for subsequent transfusions, together with occasional exchange transfusion to remove HbH, resulted in a smaller spleen size, a significant decrease in reticulocytosis, and decreased erythropoietin levels consistent with an improvement in tissue hypoxia. This approach is similar to the hypertransfusion regime employed in Thailand. However, whether the benefits of this more intensive transfusion regimen for BHFS patients outweigh the increased burden of iron chelation has yet to be investigated. Development of iron overload was documented at 6 months of age in 1 patient transfused with this regimen since birth57; this was compounded by the difficulty of chelation in infants.
Treatment of transfusional iron overload varies among institutions. Reports on iron burden are available in 27 of 53 transfusion-dependent patients, all of whom are being treated with at least 1 iron chelator. Their median pretransfusional ferritin level, documented at the median age of 7.5 years (range 0.7-31 years), is 1328 ng/mL (range 226-2157 ng/mL).
Other comorbidities of long-term survivors
Endocrinopathies similar to those in β-thalassemia major64-66 are documented in at least 6 transfusion-dependent patients, 5 of whom are older individuals (aged 7-15 years). These endocrinopathies include hypothyroidism, diabetes mellitus, delayed puberty, hypogonadism, and GH deficiency and are likely to result from iron overload in endocrine organs. Decreased bone density is documented in at least 4 patients, likely resulting from a combination of marrow expansion in response to chronic hemolysis, chronic anemia, iron toxicity as well as other endocrine complications.65-69 Cholelithiasis is reported in 1 case resulting from chronic hemolytic anemia.
Other comorbidities found in patients show no consistent association and include asthma, Graves disease, systemic lupus erythematous, gout, tricuspid valve dysplasia, premature ventricular contraction, migraine and idiopathic urticaria.
Discussion
Although the vast majority of affected fetuses with BHFS die in utero or a few hours after birth, there are at least 69 patients who have survived, reflecting improved intrauterine therapy and neonatal intensive care. Although patient numbers are limited, this is the largest study to date describing the natural history and long-term outcomes of individuals with BHFS.
Molecular diagnostic tools for DNA analysis obtained from chorionic villi sampling and less invasive procedures, such as accurate ultrasound measurement and analysis of fetal cell-free DNA in maternal blood, have been developed to detect BHFS pregnancies as early as the first trimester.8,70-75 However, a proportion of mothers are not screened, most often being diagnosed in mid to late gestation (median GA 22 weeks), or postnatally. This finding from our registry reflects a continuing need for improved public education, carrier detection, genetic counseling, and early antenatal diagnosis. Comprehensive prenatal screening is currently being implemented in some populations with a high carrier rate of α0-thalassemia deletions, including Hong Kong,76,77 Thailand,21,78 and mainland China,79 and this public health policy needs to be consistently employed and expanded.
In the event that parents wish to continue BHFS pregnancies despite the associated risks, early intrauterine intervention is likely to lead to prolonged gestation, improved Apgar scores, and shortened neonatal mechanical ventilation. Therefore, in such cases, it appears to be prudent that intrauterine treatment should be offered, where available. Intrauterine HSC transplantation has yet to be successful in fetuses with BHFS, and selection of an appropriate HSC source and optimization of in utero transplantation protocols and timing remain challenging. Most studies in the field are not yet ready for the clinic.80-83 Although intrauterine transfusion results in a less stormy neonatal course, it may not provide additional benefits to long-term growth and neurodevelopment. This observation suggests that once affected infants have survived gestation and the postnatal period, they have a similar chance to grow and develop normally regardless of prenatal history of intrauterine treatment. HSC transplantation in early life does not always correct poor growth (4/10 patients corrected) and unfavorable development (7/10 patients corrected).
It is remarkable that 10 of 22 fetuses (45%) untransfused in utero were born without hydropic features (Table 5). The factors underlying the development of hydropic abnormalities are still unclear8,61; however, infants with no hydropic features may have a greater degree of persistent expression of embryonic ζ-globin, and further detailed study is required to resolve this issue.
The high incidence of associated congenital abnormalities is also an important risk factor in BHFS pregnancies.59 The most common are urogenital and limb abnormalities, most likely resulting from early in utero hypoxia, both of which may remain as lifelong comorbidities. Urogenital and limb abnormalities are significant because such abnormalities may arise early in gestation, when currently available technologies are incapable of diagnosing BHFS8,70-74,84,85 and the affected fetuses are too small for intervention. However, advances in prenatal ultrasound assessment86 allow early detection of limb defects; this should be taken into account when counseling affected couples. Growth retardation is a major adverse long-term outcome as 15 of 39 patients (38%) have severely impaired weight and 21 of 44 (48%) have short stature in any given age group (Figure 2A). Although 11 of 55 patients (20%) at any given age suffer from significantly delayed intellectual development or developmental milestones, the remainder (44/55, 80%) have favorable neurodevelopmental outcomes in the long term (Figure 2B).
Maternal factors should also be considered, and mothers carrying hydropic fetuses have an increased risk of serious obstetric complications that may occur regardless of whether intrauterine therapies are given. Although the frequencies of most of maternal complications reported here are much lower than in previous literature (reviewed in Higgs2 ), suggesting a high standard of obstetric care in these cases, it is important for mothers to be monitored closely at antenatal clinics with experience of the management of complex pregnancies.
Most patients (77%, 53/69 patients) require lifelong transfusion. Blood transfusion according to functional Hb levels appears to improve many outcomes; however, these benefits may be outweighed by long-term transfusion-related complications. Assessment of endocrine functions and bone disease should be regularly performed.
In conclusion, this retrospective study of 69 patients with BHFS suggests that most will require lifelong transfusion, are likely to have some growth delay and short stature, and may also have developmental abnormalities and comorbidities. On the basis of this registry, more informed counseling can now be provided on the predicted outcomes of affected babies and associated maternal risks. If parents are determined to continue with a BHFS pregnancy, the management of affected infants after birth, when possible, should follow the standard care for patients with transfusion-dependent thalassemia by suppressing production of Hb Bart's and HbH combined with appropriate iron chelation. However, for most affected individuals worldwide, such management is not available. Given the intent to offer intrauterine therapy at some centers,87 we will continue and expand this registry to “fine tune” and develop guidelines for intrauterine intervention and postnatal transfusion. This work also emphasizes areas for preclinical research; in particular, to identify how the ζ-globin gene could be reactivated to cure this disease. Most importantly, the complex clinical outcomes of these patients even when treated in expert centers underscore the need for improved programs for prevention worldwide.
Acknowledgments
The authors thank David Weatherall for his ongoing encouragement and support.
This work was supported by the UK Medical Research Council, the National Institute for Health Research Oxford Biomedical Research Centre, The Henry Smith Charity, and Action Medical Research.
Authorship
Contribution: D.S., C.B., and D.R.H. initiated the registry; D.S. and C.B. reviewed the literature; The BHFS International Consortium took care of patients, provided clinical information, and recruited patients; D.S. analyzed data; D.S., C.B., and D.R.H. wrote the manuscript; and all members of the Consortium contributed to the data review and provided their comments on the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
A complete list of the members of the BHFS International Consortium collaborators appears in “Appendix.”
Correspondence: Douglas R. Higgs, MRC Molecular Hematology Unit, Weatherall Institute of Molecular Medicine, University of Oxford, John Radcliffe Hospital, Headington, Oxford OX3 9DS, United Kingdom; e-mail: doug.higgs@imm.ox.ac.uk; and Christian Babbs, MRC Molecular Hematology Unit, Weatherall Institute of Molecular Medicine, University of Oxford, John Radcliffe Hospital, Headington, Oxford OX3 9DS, United Kingdom; christian.babbs@imm.ox.ac.uk.
Appendix: study group members
The members of the BHFS International Consortium are: Duantida Songdej (Medical Research Council Molecular Haematology Unit, Weatherall Institute of Molecular Medicine, University of Oxford, Oxford, United Kingdom; and Division of Hematology/Oncology, Department of Pediatrics, Faculty of Medicine, Ramathibodi Hospital, Mahidol University, Bangkok, Thailand); Ali Amid (Division of Haematology/Oncology, Department of Paediatrics, Hospital for Sick Children, University of Toronto, Toronto, ON, Canada); Vip Viprakasit (Department of Pediatrics & Thalassemia Centre, Faculty of Medicine, Siriraj Hospital, Mahidol University, Bangkok, Thailand); Sylvia Titi Singer (Department of Hematology-Oncology, UCSF Benioff Children's Hospitals Oakland, Oakland, CA); Elliott P. Vichinsky (Department of Hematology-Oncology, UCSF Benioff Children's Hospitals Oakland, Oakland, CA); Vivian Chan (Department of Medicine, Queen Mary Hospital, University of Hong Kong, Hong Kong, China); A. W. Leung (Department of Paediatrics, Prince of Wales Hospital, The Chinese University of Hong Kong, Shatin, Hong Kong, China); C. K. Li (Department of Paediatrics, Prince of Wales Hospital, The Chinese University of Hong Kong, Shatin, Hong Kong, China); S. Y. Ha (Department of Pediatrics & Adolescent Medicine, The University of Hong Kong, Queen Mary Hospital, Hong Kong, China); Melanie Kirby-Allen (Division of Haematology/Oncology, Department of Paediatrics, Hospital for Sick Children, University of Toronto, Toronto, ON, Canada); Daniel E. Bauer (Division of Hematology/Oncology, Boston Children's Hospital, Department of Pediatric Oncology, Dana-Farber Cancer Institute, Harvard Stem Cell Institute, Department of Pediatrics, Harvard Medical School, Boston, MA); Seoh Leng Yeoh (Department of Pediatrics, Hospital Pulau Pinang, George Town, Malaysia); Lydia H. Pecker (Center for Cancer and Blood Disorders, Children's National Medical Center, Washington, DC; and Department of Pediatrics, The George Washington University School of Medicine and Health Sciences, Washington, DC); Uma Athale (Division of Haematology/Oncology, McMaster Children's Hospital, Hamilton, ON, Canada); John Wu (Division of Hematology and Oncology, Department of Pediatrics, BC Children's Hospital, University of British Columbia, Vancouver, BC, Canada); Sule Unal (Division of Pediatric Hematology, Department of Pediatrics, Faculty of Medicine, Hacettepe University, Ankara, Turkey); Fatma Gumruk (Division of Pediatric Hematology, Department of Pediatrics, Faculty of Medicine, Hacettepe University, Ankara, Turkey); Irene Roberts (Medical Research Council Molecular Haematology Unit, Weatherall Institute of Molecular Medicine, University of Oxford, Oxford, United Kingdom); Vineet Bhandari (Division of Perinatal Medicine, Department of Pediatrics, Yale Child Health Research Center, Yale University School of Medicine, New Haven, CT; and St. Christopher's Hospital for Children/Drexel University College of Medicine, Philadelphia, PA); Peter F. Coccia (Division of Pediatric Hematology-Oncology, Department of Pediatrics, University of Nebraska Medical Center, Omaha, NE); Christian Babbs (Medical Research Council Molecular Haematology Unit, Weatherall Institute of Molecular Medicine, University of Oxford, Oxford, United Kingdom); and Douglas R. Higgs (Medical Research Council Molecular Haematology Unit, Weatherall Institute of Molecular Medicine, University of Oxford, Oxford, United Kingdom).