In this issue of Blood, Du and colleagues describe a novel approach to eradicate the malignant cells of classical Hodgkin lymphoma by a combination of differentiation therapy and targeting the restored B-cell phenotype in preclinical cell line models.1
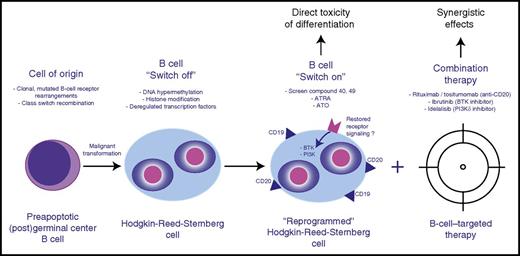
Therapeutic design of combined cell differentiation and B-cell–targeted therapy. BTK, Bruton’s tyrosine kinase.
Therapeutic design of combined cell differentiation and B-cell–targeted therapy. BTK, Bruton’s tyrosine kinase.
Classical Hodgkin lymphoma (cHL) is characterized by the presence of rare malignant Hodgkin-Reed-Sternberg (HRS) cells in a quantitatively dominant microenvironment mainly composed of a multitude of different nonmalignant immune cells. The most prominent molecular hallmarks of HRS cells described to date include aberrant signaling pathways (eg, NF-κB and JAK-STAT signaling), immune escape phenotypes, a unique sectretome shaping the composition of an immunomodulatory microenvironment, and the loss of lineage-specific expression markers.2 In a subset of cases, latent Epstein-Barr virus infection contributes to these hallmarks. As sequencing studies have shown HRS cells to be of B-cell origin and, more specifically, to be derived from preapoptotic germinal center B cells, this remarkable loss of a B-cell–specific expression program has been extensively studied in the past, and deciphering the contributing factors to this phenotype is considered paramount to understanding cHL pathogenesis in general.3 Among the most important and widely discussed mechanisms of HRS cell reprogramming are promoter hypermethylation, aberrant histone modifications, and altered expression of key B-cell differentiation factors that in conjunction might lead to lineage-inappropriate gene expression profiles.4 However, in the past, these specific aspects of HRS cell biology have been rarely brought into the context of therapeutic considerations.
In the current paper, Du et al explore the therapeutic potential of restoring the lost B-cell phenotype of HRS cells. The authors used an elegant pharmacological screen (28 160-compound library) in various cHL-derived cell lines (L1236, L428, and KM-H2) in which CD19 promoter activity was measured by luciferase reporter constructs. This screen identified 3 compounds that significantly increased CD19 expression in these cell lines. Interestingly, although the exact mechanisms remain to be determined, 2 of these compounds (compounds 40 and 49) were previously reported to interfere with epigenetic regulators and led to transcription-activating histone H3 modifications in the present study.1 The authors also tested 2 agents, all-trans retinoic acid (ATRA) and arsenic trioxide (ATO), that are well established in differentiation therapy of acute promyelocytic leukemia5 and found similar B-cell phenotype restoring properties to the compounds of the pharmacological screen. Of note, a previous study using cHL-derived cell lines established that ATO resulted in increased apoptosis through downregulated NF-κB activity.6 This raises the possibility that the observed increase in CD19/CD20 expression, and downregulated NF-κB pathway activity, are linked as part of a more global B-cell expression program that can be pharmacologically restored. Although it remains to be determined to what extend the various tested drugs can partially or even fully restore the lost B-cell phenotype, the presented data firmly establish direct in vitro toxicity using differentiation therapy.
The authors proceed to address another important question with therapeutic relevance: Does the restoration of the B-cell phenotype render the HRS cells sensitive to novel therapies targeting B-cell biology? Specifically, they explore the effects of anti-CD20 therapeutic antibodies and targeting of B-cell receptor signaling kinases in conjunction with ATO or compound 40 (see figure). Despite individual reports of clinical activity in cHL, most studies testing rituximab (anti-CD20), idelalisib (phosphatidylinositol 3-kinase P110δ [PI3K-P110δ] inhibitor), and ibrutinib (BTK inhibitor) have been rather disappointing, and none of these drugs as single agents are anticipated to play a major role in clinical management of cHL.7,8 Therefore, the preclinical rationale to search for a “sensitizer” to these agents appears attractive. The presented in vitro studies demonstrate significantly decreased viability and enhanced cytotoxicity in L1236 and L428 cells treated with anti-CD20 therapeutic antibodies (rituximab and tositumomab) when cells were preincubated with ATRA or ATO. This effect could be further enhanced by addition of compound 40. However, the therapeutic effects of antibodies in cell line monoculture systems are inherently difficult to interpret, and immunocompetent in vivo models of cHL are lacking to dissect the differential effects of antigen-dependent cellular cytotoxicity and complement-mediated cytolysis. To an even larger extent, synergistic effects, measured by decreased viability across a wide range of cHL-derived cell lines, were observed with combination therapy of ATO/compound 40 and ibrutinib or idelalisib. Moreover, the authors show that ATO and compound 40 significantly increased CD19/CD20 expression as well as BTK and AKT phosphorylation, indicative of restored B-cell receptor and PI3K pathway signaling. Despite these convincing in vitro results, further studies are needed, as it remains an open question how exactly ibrutinib or idelalisib exerts its therapeutic activity, considering that HRS cells likely do not have the capacity to express functional, high-affinity B-cell receptors3 and it is unclear how HRS cells might regain pathway addiction through differentiation therapy in vivo.
Overall, the presented study provides convincing proof of concept that pharmacological restoration of the B-cell phenotype provides valuable insight into cHL pathogenesis. However, the clinical utility of the identified compounds and overall strategy might be less certain and will be, in part, tied to host toxicity profiles. The landscape of cHL clinical management is rapidly evolving with the introduction of brentuximab vedotin and checkpoint inhibitors as US Food and Drug Administration–approved drugs and with extensive testing in randomized clinical trials focusing on various time points of patient management.9 Anticipated treatment changes might limit the room for additional concepts to be tested. However, it is worth noting that limiting treatment-related toxicity is still an underserved clinical need in cHL, and approaches that enable more targeted, hopefully less toxic therapies should still be explored with priority.
Conflict-of-interest disclosure: The author declares no competing financial interests.