To the editor:
Langerhans cell histiocytosis (LCH) has been described as an inflammatory myeloid neoplasia that affects various organs. Central nervous system involvement may result in endocrinopathies and progressive neurodegeneration (ND-LCH), characterized by low-grade inflammation and degenerative changes, resulting in cognitive deficits, behavioral disturbances, and neuromotor dysfunction.1 Prolonged disease activity is a risk factor for ND-LCH.1 Potential biomarkers in cerebrospinal fluid (CSF) indicative of ND-LCH have been suggested (eg, neurofilament protein light chain [NF-L]),2 and increased interleukin-17A (IL-17A) levels in blood and extracranial lesions of LCH patients have been associated with local and systemic inflammation.3,4 Independently, IL-17A has been shown to disrupt the blood-brain barrier and increase production of reactive oxygen species in brain endothelial cells, pointing toward a crucial step for the development of experimental autoimmune encephalitis5 and possibly also for ND-LCH.
There is currently no established therapy for ND-LCH, although many have been proposed.6-9 We aimed to find an effective therapy for a child with severe, rapidly progressive ND-LCH unresponsive to standard treatments. The patient was followed clinically (for >15 years), by magnetic resonance imaging (MRI) and by measuring NF-L in the CSF and later also IL-17A in CSF and plasma.
Briefly, the patient was diagnosed with LCH at 5 months of age, which rapidly progressed into multisystem LCH with risk-organ involvement. Following initial treatment (LCH-II protocol) she stabilized, but after several reactivations, she developed a chronic active disease with elevated erythrocyte sedimentation rate (ESR). At 4 years of age, clinical signs of ND were noted (tripping, clumsiness), and brain MRI a year later revealed widespread ND-LCH affecting the basal ganglia, corpus callosum, cerebellum, and medulla. Despite many treatments, the patient deteriorated markedly within the next 4 years. Prompted by the need for effective treatment, at the age of 8 years we initiated granulocyte-and-monocyte apheresis (GMA), initially in parallel with conventional LCH treatment (Figure 1; at week 0), aiming at removing activated granulocytes and monocytes, thus reducing the inflammatory load.10
GMA treatment resulted in decreased inflammation in blood and CSF. (A) Description of disease status in relation to age and treatments over time. GMA (int) indicates the interval of GMA in weeks for the indicated period, as specified by the numbers inside the boxes in the GMA row. GMA (week) indicates the number of weeks from GMA onset (0), specified by the numbers in italics below the boxes. For MRI, ND, and NF-L, the dashes indicate unchanged status compared with previous examination. The arrowheads pointing down indicate regression/decreased levels compared with the previous examination, whereas arrowheads pointing up indicate increased levels compared with the previous examination. The dashed lines next to each treatment indicate the treatment period. (B) NF-L and corresponding IL-17A protein levels in CSF. Results from only 1 NF-L enzyme-linked immunosorbent assay kit (from week 109 onward) are shown for simplicity because of different reference values and sensitivity of the 2 NF-L enzyme-linked immunosorbent assays used (see supplemental Materials and methods). IL-17A protein levels in plasma (C) and the percentages of IL-17A+ monocytes in peripheral blood mononuclear cells (PBMCs) (D) measured from week 115 onward. The interval of GMA in weeks is indicated in the boxes on top of the graphs in panels B-D. The dotted line indicates the mean value of controls (healthy), and the dashed line indicates +3 standard deviations from control mean. IL-17A protein levels in plasma (E) and percentage of IL-17A+ monocytes in PBMCs (F) in relation to the frequency of GMA. (G) Relative changes in plasma levels of IL-17A and IL-23 on weeks 326 (7 weeks before GMA termination) and 416 (18 months after GMA termination) as compared with week 115. CST, corticosteroids; DI, diabetes insipidus; PPBS, posterior pituitary bright spot; 6-MP, 6-mercaptopurine. The ESR values are presented as the mean value for the indicated period.
GMA treatment resulted in decreased inflammation in blood and CSF. (A) Description of disease status in relation to age and treatments over time. GMA (int) indicates the interval of GMA in weeks for the indicated period, as specified by the numbers inside the boxes in the GMA row. GMA (week) indicates the number of weeks from GMA onset (0), specified by the numbers in italics below the boxes. For MRI, ND, and NF-L, the dashes indicate unchanged status compared with previous examination. The arrowheads pointing down indicate regression/decreased levels compared with the previous examination, whereas arrowheads pointing up indicate increased levels compared with the previous examination. The dashed lines next to each treatment indicate the treatment period. (B) NF-L and corresponding IL-17A protein levels in CSF. Results from only 1 NF-L enzyme-linked immunosorbent assay kit (from week 109 onward) are shown for simplicity because of different reference values and sensitivity of the 2 NF-L enzyme-linked immunosorbent assays used (see supplemental Materials and methods). IL-17A protein levels in plasma (C) and the percentages of IL-17A+ monocytes in peripheral blood mononuclear cells (PBMCs) (D) measured from week 115 onward. The interval of GMA in weeks is indicated in the boxes on top of the graphs in panels B-D. The dotted line indicates the mean value of controls (healthy), and the dashed line indicates +3 standard deviations from control mean. IL-17A protein levels in plasma (E) and percentage of IL-17A+ monocytes in PBMCs (F) in relation to the frequency of GMA. (G) Relative changes in plasma levels of IL-17A and IL-23 on weeks 326 (7 weeks before GMA termination) and 416 (18 months after GMA termination) as compared with week 115. CST, corticosteroids; DI, diabetes insipidus; PPBS, posterior pituitary bright spot; 6-MP, 6-mercaptopurine. The ESR values are presented as the mean value for the indicated period.
GMA was initially performed weekly for 10 weeks, followed by clinical improvement, decreased NF-L, and regression of cerebellar lesions. After a 10-week break, GMA was restarted and performed every second week (11 sessions). During a subsequent 4-month break, higher NF-L levels were noted, and GMA was restarted but performed less frequently and with fewer noticeable benefits. Therefore, her treatment was replaced with a combination of vinblastine and simvastatin (without GMA) to benefit from the anti-inflammatory effect of simvastatin. However, the patient deteriorated the following 6 months with more fatigue, impaired balance and gait, and increased NF-L (Figure 1A). Nystagmus was confirmed a few months later. Given the positive effects of the first GMA treatments, GMA was restarted (week 115). Cytarabine was given in parallel for 6 months based on positive reports from others.11 Because of absence of clinical improvement and lack of alternative treatment, and considering the positive effects of natalizumab on patients with multiple sclerosis,12 we then administered natalizumab after thorough discussions with neurologists and the parents. GMA continued once every 4 weeks. Overall the patient tolerated the treatment well. After 6 months of natalizumab treatment, NF-L levels were lower, but at the 1-year follow-up, NF-L had increased again (Figure 1B; weeks 195 and 216, respectively), walking had deteriorated, and ESR indicated active inflammation (Figure 1A). Because of lack of improvement and increased risk of progressive multifocal leukoencephalopathy with prolonged treatment, natalizumab was ceased.
Attracted by the lack of side effects from GMA in this patient who had not responded to other treatments, GMA was now (week 220) intensified to once weekly in parallel to low corticosteroid pulses (initially every 2 weeks, later every 3-4 weeks) for >2 years. NF-L decreased during this period, and the neurological deterioration eventually slowed down; the patient stabilized clinically, and MRI remained stable. In addition, but most apparent initially, positive effects were noted on the patient’s fatigue, possibly because of the marked decrease in circulating cytokine levels (Figure 1B-G).
The impact of GMA over time on IL-17A in CSF, in plasma, and inside blood monocytes is illustrated in Figure 1B, C, and D, respectively. The methods used are described in the supplemental Materials and methods (available on the Blood Web site). When GMA was performed weekly, both the plasma IL-17A levels (Figure 1E) and the percentage of IL-17A+ monocytes in blood (Figure 1F) tended to be lower, compared with less frequent treatment, but the influence of spontaneous improvement or parallel treatments on this outcome cannot be ruled out. A remarkable reduction was observed in IL-17A and IL-23 plasma levels during the last years (Figure 1G; week 326), whereas ESR remained slightly elevated. The increased IL-17A production on week 242 was accompanied by increased ESR and IL-23 levels, maybe because of subclinical infection. Stable low IL-17A plasma levels for the last 2 years and apparent neurological stabilization suggested less value from future GMA. Because of this and emerging difficulties with vascular access, GMA was stopped on week 333 after 169 sessions. At 6- and 18-month follow-up after GMA termination, NF-L and IL-17A levels in CSF remained low (Figure 1B) as did plasma levels of IL-17A and IL-23 (Figure 1G; week 416).
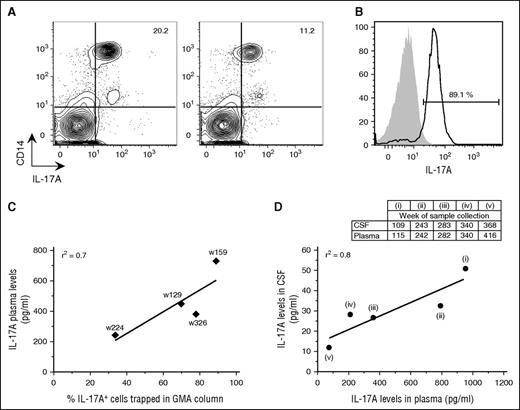
Analysis of blood samples before and immediately after GMA showed 50% reduction in the percentage of circulating IL-17A+ monocytes in the patient’s blood immediately after treatment (Figure 2A). Notably, up to 90% of PBMCs captured in the GMA column were IL-17A+ monocytes (Figure 2B), and a positive correlation was observed between plasma IL-17A levels and percentage of IL-17A+ monocytes in the GMA column (Figure 2C), as well as between IL-17A levels in plasma and CSF (Figure 2D).
GMA successfully removed IL-17A-producing monocytes from blood. (A) Contour plots of PBMCs that were isolated from the patient’s blood on week (w) 159 before and immediately after GMA were stained intracellularly with an anti-IL-17A antibody in combination with surface marker staining for monocytes (CD14). (B) Histograms of the cells that were trapped in the GMA column on week 159 and stained with antibodies for either IL-17A (black line) or Alexa 647 isotype (gray area). The percentage refers to IL-17A+ cells among PBMCs. (C) Correlation between plasma IL-17A levels and percentage of IL-17A+ cells trapped in the GMA column. (D) Correlation between IL-17A levels in CSF and plasma. CSF was collected 1 to 2 times per year and mostly on occasions separate from when plasma was collected; therefore, panel D includes only 5 time points: 1 time point following a long break in GMA (i), 3 time points with plasma and CSF collection at maximum 1 week apart (ii-iv), and 1 time point ≥6 months after GMA termination (v).
GMA successfully removed IL-17A-producing monocytes from blood. (A) Contour plots of PBMCs that were isolated from the patient’s blood on week (w) 159 before and immediately after GMA were stained intracellularly with an anti-IL-17A antibody in combination with surface marker staining for monocytes (CD14). (B) Histograms of the cells that were trapped in the GMA column on week 159 and stained with antibodies for either IL-17A (black line) or Alexa 647 isotype (gray area). The percentage refers to IL-17A+ cells among PBMCs. (C) Correlation between plasma IL-17A levels and percentage of IL-17A+ cells trapped in the GMA column. (D) Correlation between IL-17A levels in CSF and plasma. CSF was collected 1 to 2 times per year and mostly on occasions separate from when plasma was collected; therefore, panel D includes only 5 time points: 1 time point following a long break in GMA (i), 3 time points with plasma and CSF collection at maximum 1 week apart (ii-iv), and 1 time point ≥6 months after GMA termination (v).
Although LCH pathogenesis remains unclear, increased IL-17A production has been associated with higher disease activity.3,4 Our observation that high IL-17A levels in blood and CSF accompany the disease progression supports a possible role for IL-17A in ND-LCH pathogenesis. Notably, when IL-17A levels decreased, NF-L levels also decreased and the patient’s fatigue improved. Measurement of IL-17A plasma levels in 3 additional LCH patients described to have isolated central nervous system involvement also revealed significantly increased IL-17A plasma levels (394, 369, and 234 pg/mL, with corresponding ESR of 3, 5, and 26 mm/h, respectively) compared with 28 controls (median IL-17A: 120 pg/mL; P < .01, Mann-Whitney U test). Similarly, high IL-17A and IL-23 levels have been reported in serum and CSF of patients with amyotrophic lateral sclerosis, a progressive neurodegenerative disease.13
Only a few children with either acute relapse of multiple sclerosis, neuromyelitis optica, or acute disseminated encephalomyelitis have been reported to receive short-term apheresis treatment.14 Recently, short-term GMA has also been beneficial in sarcoidosis.15 To the best of our knowledge, our study is the first in which GMA has been used regularly for long-term (>6 years) and, importantly, without major side effects. The treatment removed cytokine-producing cells from blood and decreased the levels of IL-17A in plasma and CSF. Possibly, the number of cytokine-producing cells in tissues was also reduced.16 Because various treatments were administered and the natural development of the neurodegenerative process is not well known, where some patients stabilize with age, we cannot be certain if, and if so to what extent, GMA contributed to the stabilization of our patient. Nevertheless, our study suggests that (long-term) GMA may be considered for experimental treatment in patients with systemic LCH, aiming to reduce hypercytokinemia and disease activity. Finally, our novel finding that IL-17A may be used as an additional CSF biomarker for ND-LCH provides an additional method for detecting and monitoring ND-LCH.
The online version of this article contains a data supplement.
Authorship
Acknowledgments: The authors thank Ingrid van't Hooft Hagberg at Karolinska Institutet for providing detailed information on the neuropsychological evaluations of the patient, Anna Fogdell-Hahn at Karolinska Institutet for providing control CSF samples, the Clinical Neurochemistry Laboratory in Gothenburg for performing the NF-L analyses, and Puran Chen at Karolinska Institutet for helping illustrate the timeline changes of the patient’s condition. This work was supported by grants from the Swedish Children’s Cancer Foundation, the Swedish Research Council, Karolinska Institutet, and the Stockholm County Council (ALF project) (J.-I.H. and M.S.); the Cancer and Allergy Foundation of Sweden (J.-I.H.); and Karolinska Institutet (M.L.). M.L. also received postdoc scholarships from Karolinska Institutet, Mary Béve Foundation, and Märta and Gunnar V Philipsons Foundation.
Contribution: M.L. designed and performed the experiments, analyzed the data, interpreted the results, and prepared the figures; S.O.-Å. cared for the patient, provided blood samples and clinical data, and contributed to interpretation of the results and figure layout; D.G. arranged for NF-L measurements in CSF, recorded the neurological status of the patient over time, and contributed to interpretation of the results and figure layout; U.A.N. supervised the GMA treatments in Stockholm; G.B. supervised the GMA treatments in Linköping and retrieved the ESR values for that period; E.L. reviewed the MRI examinations of the patient; T.v.B.G. cared for the patient and provided blood and CSF samples; M.S. supervised the study and provided feedback on the experimental setup, the analysis and interpretation of the results, and figure layout; J.-I.H. cared for the patient, initiated all the treatments described in the manuscript, provided blood and CSF samples, contributed to interpretation of the results, and generally supervised the study; M.L. and S.O.-Å. wrote the manuscript; and all authors contributed to editing and finalizing the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Magda Lourda, Center for Infectious Medicine, F59, Department of Medicine, Karolinska Institutet, Karolinska University Hospital, Huddinge, 14186 Stockholm, Sweden; e-mail: magdalini.lourda@ki.se; and for clinical questions Selma Olsson-Åkefeldt, Childhood Cancer Research Unit, Q6:05, Astrid Lindgren Children’s Hospital, 17176 Stockholm, Sweden; e-mail: selma.olsson-akefeldt@karolinska.se.