Key Points
Defect in thrombus formation, platelet aggregation, and ADP secretion induced by ablation or inhibition of SERCA3−/−.
Abstract
The role of the sarco-endoplasmic reticulum calcium (Ca2+) adenosine triphosphatase (ATPase) 3 (SERCA3) in platelet physiology remains poorly understood. Here, we show that SERCA3 knockout (SERCA3−/−) mice exhibit prolonged tail bleeding time and rebleeding. Thrombus formation was delayed both in arteries and venules in an in vivo ferric chloride–induced thrombosis model. Defective platelet adhesion and thrombus growth over collagen was confirmed in vitro. Adenosine 5′-diphosphate (ADP) removal by apyrase diminished adhesion and thrombus growth of control platelets to the level of SERCA3−/− platelets. Aggregation, dense granule secretion, and Ca2+ mobilization of SERCA3−/− platelets induced by low collagen or low thrombin concentration were weaker than controls. Accordingly, SERCA3−/− platelets exhibited a partial defect in total stored Ca2+ and in Ca2+ store reuptake following thrombin stimulation. Importantly ADP, but not serotonin, rescued aggregation, secretion, and Ca2+ mobilization in SERCA3−/− platelets, suggesting specificity. Dense granules appeared normal upon electron microscopy, mepacrine staining, and total serotonin content, ruling out a dense granule defect. ADP induced normal platelet aggregation, excluding a defect in ADP activation pathways. The SERCA3-specific inhibitor 2,5-di-(tert-butyl)-1,4-benzohydroquinone diminished both Ca2+ mobilization and secretion of control platelets, as opposed to the SERCA2b inhibitor thapsigargin. This confirmed the specific role of catalytically active SERCA3 in ADP secretion. Accordingly, SERCA3-dependent Ca2+ stores appeared depleted in SERCA3−/− platelets. Finally, αIIbβ3 integrin blockade did not affect SERCA3-dependent secretion, therefore proving independent of αIIbβ3 engagement. Altogether, these results show that SERCA3-dependent Ca2+ stores control a specific ADP secretion pathway required for full platelet secretion induced by agonists at low concentration and independent of αIIbβ3.
Introduction
Among regulatory mechanisms of Ca2+ intracellular signaling in platelets, the sarco-endoplasmic Ca2+ adenosine triphosphatases (SERCAs) that pump Ca2+ into intracellular stores are particularly relevant.1 SERCAs are encoded by 3 genes, ATP2A1, ATP2A2, and ATP2A3, which produce several alternate transcripts and protein isoforms: SERCA1a/b, SERCA2a-c, and SERCA3a-f. They are found in multiple tissues, but platelets exhibit only SERCA2b and SERCA3 isoforms.2-4 SERCAs maintain a Ca2+ concentration gradient between the cytosol (100 nM) and the endoplasmic reticulum (1 mM), requiring degradation of adenosine triphosphate (ATP)5 into adenosine 5′-diphosphate (ADP) then released into the cytosol.6
SERCA enzymes share similar structures with distinct intrinsic activities: higher Ca2+ affinity for SERCA2b than for SERCA3 (K1/2 ∼0.27 µM vs 1 µM) but a lower Ca2+ uptake (7 nmol/min per mg of protein vs 21 nmol/min per mg)7,8 allowing cytosolic Ca2+ to be maintained at low levels in the resting cells.9
Pathologies and mouse models provide insight into SERCA2b and SERCA3 functions. Mutations in the human ATP2A2 gene affecting SERCA2 lead to Darier syndrome in humans, a dermatological syndrome.10,11 SERCA mutations are associated with some cancers,12-14 suggesting involvement in cell differentiation.15 SERCA3 human mutations seem associated with type 2 diabetes.16 Mouse SERCA2 knockouts are not viable at the homozygous state, but heterozygotes exhibit SERCA2a-type (defect in heart contractility and relaxation)17 and SERCA2b-type defects, evocative of Darier syndrome.18 Mouse SERCA3 knockout (SERCA3−/−) mice exhibit no phenotypic alterations,19 except for an altered gustatory nerve response.20 Impaired relaxation of SERCA3−/− aorta rings was reported, with defective relaxation of vascular smooth muscle cells, altered Ca2+ signaling, and low nitric oxide production.19 In vitro, low insulin secretion and altered Ca2+ oscillations were reported.21-23 Altogether these results point to a potential specific role for SERCA3 in Ca2+ signal modulation.
Among other differences in platelets is a different topology, peripheral for SERCA3, more central for SERCA2b.24 SERCA3 is specifically associated with acidic Ca2+ stores,25 and with STIM1 (the Ca2+ sensor of the store operated Ca2+ entry [SOCE]).26 To assess the role of SERCA3 in platelets, we have decided to assess the hemostasis status of SERCA3−/− mice. Here, we report the analysis of both in vivo and in vitro hemostasis features of SERCA3−/− mice and show that ablation of SERCA3 lowers in vivo hemostatic and thrombotic responses, as well as platelet adhesive and secretory functions in vitro. Moreover, we find that thrombin or collagen activation is affected because of low dense granule secretion. Importantly, mobilization and secretion are rescued by ADP addition, consistent with a role for SERCA3 in ADP secretion. Confirming a specific role for SERCA3, pharmacological inhibition of SERCA3, but not of SERCA2b, in control platelets recapitulates the same defect in secretion in vitro, definitely pointing to SERCA3 as specifically involved in the regulation of such a secretion. Finally, SERCA3-dependent secretion appears independent of αIIbβ3 integrin engagement. These results thus point to an as yet unreported role of SERCA3 in hemostasis and in positive regulation of platelet dense granule and ADP secretion.
Materials and methods
Material
Fibrillar collagen (equine type I) and ADP were obtained from Kordia (Leiden, The Netherlands). Apyrase (grade 7), rhodamine 6G, bovine thrombin, ferric chloride, indomethacin, and the SERCA inhibitors thapsigargin (Tg) and 2,5-di-(tert-butyl)-1,4-benzohydroquinone (tBHQ) were obtained from Sigma (St. Louis, MO). We purchased d-Phe-Pro-Arg chloromethylketone dihydrochloride from Calbiochem-VWR (Fontenay-sous-Bois, France). The protease-activated receptor (PAR) agonist peptide (PAR4-AP; AYPGKF-NH2) was purchased from Bachem (Weil am Rheim, Germany). Mepacrine (quinacrine dihydrochloride) was from Sigma-Aldrich (St. Louis, MO). Alexa Fluor 488-labeled phalloidin was from Invitrogen (Cergy Pontoise, France). Phycoerythrin-labeled rat anti-mouse integrin αIIbβ3 monoclonal antibody (mAb; JON/A), fluorescein isothiocyanate–labeled rat anti-mouse CD62P (P-selectin) mAb (Wug.E9) and purified rat anti-mouse integrin αIIbβ3 mAb (Leo.H4) were from Emfret Analytics (Würzburg, Germany). Oregon Green 488 BAPTA1-AM was from Molecular Probes (Eugene, OR). Polyclonal antibodies specific for SERCA2b27 and for SERCA3 (N89)28 were generous gifts from F. Wuytack (Katholieke Universiteit Leuven, Leuven, Belgium). The antibody directed against 14-3-3ζ, was obtained from Santa Cruz Biotechnology (Santa Cruz, CA). Phospho-(Ser) protein kinase C substrate antibody was from Cell Signaling Technology (Danvers, MA).
SERCA3−/− mice.
The Black Swiss SERCA3−/− mice originally generated by G. E. Shull (University of Cincinnati, OH)19 were crossed with C57BL/6 mice and kindly provided by P. Gilon (University of Louvain, Belgium) with the authorization of G. E. Shull. Wild-type (WT) littermate mice were provided as well and served as controls. Transferred wt/- heterozygous mice were intercrossed and homozygotes were detected by polymerase chain reaction, using published oligonucleotide primers.19 All experimental procedures were carried out in accordance with the European legislation concerning the use of laboratory animals and approved by the Animal Care and Ethical Committee of Université Paris-Sud (agreement #00243-02).
Hematologic analysis and bleeding time.
Blood counts were determined with an automatic cell counter (scil Vet abc Plus; Horiba Medical, France). Bleeding time assays were performed as previously described,29 on 8- to 12-week-old mice.
Measurement of intracellular calcium.
Mouse platelets (3 × 107 platelets per mL) were loaded with the Ca2+-sensitive dye Oregon Green 488 BAPTA1-AM (1 mM) for 45 minutes at 20°C. Ca2+ mobilization induced by thrombin was analyzed in Ca2+ free medium and in the presence of 0.5 mM EGTA using an Accuri C6 flow cytometer. Changes in Ca2+ signal intensity were calculated as the ratios of fluorescence of activated over nonactivated platelets and the area below the curve for 2 minutes after agonist addition was chosen as an indicator of the calcium response. Fluorescence calibration in nanomolar Ca2+ was established as described in the supplemental Methods (available on the Blood Web site).
In vitro thrombus formation under flow conditions.
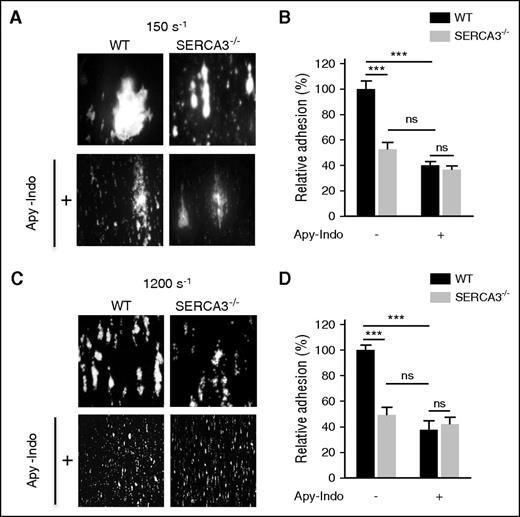
Thrombus formation was evaluated in a whole blood perfusion assay on a fibrillar collagen matrix (50 μg/mL) at various shear rates (150 s−1 and 1200 s−1) and recorded and analyzed as previously described.29 Thrombus formation was evaluated by assessment of platelet adhesion quantitated by measurement of the mean percentage of the total area covered by thrombi.
Ferric chloride–induced thrombosis model.
Ferric chloride (FeCl3) injury was induced in 4- to 5-week-old mice, as previously described.30 Briefly, rhodamine 6G (3.3 mg/kg) was injected into the retro-orbital plexus of anesthetized mice (to label platelets). After topical deposition on the mesenteric vessels of FeCl3 solution (10%), thrombus growth was monitored in real-time with an inverted epifluorescent microscope (×10) (Nikon Eclipse TE2000-U).
Serotonin assay.
Platelet serotonin (5HT) was assessed using the Serotonin Enzyme-Linked Immunosorbent Assay Kit from Abcam (Cambridge, UK). The 5HT was assayed in platelets supernatants or in platelet lysate (after 4 freeze-thaw cycles) to determine platelet total 5HT content.
Statistical analysis.
Statistical significance was evaluated with the Student t tests or 1-way analysis of variance (ANOVA) followed by the Tukey pairwise test as indicated, using GraphPad Prism (San Diego, CA).
Results
Prolonged bleeding time and delayed in vivo thrombosis in SERCA3−/− mice
We first confirmed in our mouse colony the deletion of Atp2a3, the mouse SERCA3 gene, by polymerase chain reaction (data not shown) and the absence of the SERCA3 protein in platelets by western blotting (Figure 1A). Note that platelet SERCA2b is expressed at the same level as controls. The tail clipping bleeding time assay was found significantly increased in SERCA3−/− vs WT mice (142 ± 12 s for SERCA3−/− mice vs 67 ± 5 s for controls, P < .001; Figure 1B). Moreover, a marked rebleeding tendency was noted with 75% rebleeding within 1 minute of bleeding arrest vs 10% for controls (Figure 1C). Platelets were normal morphologically (Figure 1D), slightly larger in size (5.82 ± 0.08 compared with 4.99 ± 0.04 fL; Table 1), and in slightly lower numbers than controls (average 778.2 ± 0.31 × 109/L vs 914.5 ± 0.33 × 109/L, n = 21 and 18, respectively; Table 1), not low enough to explain the prolonged bleeding time. Other blood cell counts were normal.
Characterization of hemostasis and in vivo thrombosis in SERCA3−/− mice. (A) Western blot of SERCAs in mouse platelets. Control (WT) and SERCA3−/− mouse platelets were collected and solubilized and subjected to sodium dodecyl sulfate polyacrylamide gel electrophoresis, prior to transfer to nitrocellulose and detection by antibodies specific for SERCA3 or SERCA2b.28 After addition of a secondary antibody coupled to horse radish peroxidase, bands were revealed by chemiluminescence. The 14-3-3ζ adaptor was used as an internal standard for normalization. Note the absence of SERCA3 in SERCA3−/− platelets and the same levels of SERCA2b in both control and SERCA3−/− platelets. (B) Tail bleeding time. Tail bleeding was performed as indicated in “Materials and methods,” and bleeding time assessed both on control (WT) and SERCA3−/− mice. Results are presented as mean ± SEM, using the Student t test; ***P < .001. (C) Rebleeding was assessed for 1 minute following initial bleeding arrest. A total of 18 control and 21 SERCA3−/− mice were used. (D) Transmission electron microscopy of resting control and SERCA3−/− platelets. Platelets were subjected to standard transmission electron microscopy. Upper panel, control (WT); lower panel, SERCA3−/− platelets. The scale bar (0.5 µm) is shown in the lower left corner of the WT panel. (E) Kinetics of in vivo ferric chloride-induced thrombosis of mesenteric vessels. Venules (v) or arterioles (a) are shown by fluorescence microscopy (limits outlined with white dashed lines), thrombi being visualized by rhodamine 6G–labeled platelets. Images at 0, 30, and 60 minutes are shown. (F) Quantification of thrombus formation. Time to occlusion was noted for 18 control (WT, closed circles) and 21 SERCA3−/− (open circles) mice up to 60 minutes, the maximal time assessed. Results were analyzed using 1-way ANOVA followed by Tukey’s multiple comparison test; ***P < .001. (G) Quantification of emboli. The number of emboli shedding from thrombi was assessed for 60 minutes, both in venules and arterioles of control and SERCA3−/− mice.
Characterization of hemostasis and in vivo thrombosis in SERCA3−/− mice. (A) Western blot of SERCAs in mouse platelets. Control (WT) and SERCA3−/− mouse platelets were collected and solubilized and subjected to sodium dodecyl sulfate polyacrylamide gel electrophoresis, prior to transfer to nitrocellulose and detection by antibodies specific for SERCA3 or SERCA2b.28 After addition of a secondary antibody coupled to horse radish peroxidase, bands were revealed by chemiluminescence. The 14-3-3ζ adaptor was used as an internal standard for normalization. Note the absence of SERCA3 in SERCA3−/− platelets and the same levels of SERCA2b in both control and SERCA3−/− platelets. (B) Tail bleeding time. Tail bleeding was performed as indicated in “Materials and methods,” and bleeding time assessed both on control (WT) and SERCA3−/− mice. Results are presented as mean ± SEM, using the Student t test; ***P < .001. (C) Rebleeding was assessed for 1 minute following initial bleeding arrest. A total of 18 control and 21 SERCA3−/− mice were used. (D) Transmission electron microscopy of resting control and SERCA3−/− platelets. Platelets were subjected to standard transmission electron microscopy. Upper panel, control (WT); lower panel, SERCA3−/− platelets. The scale bar (0.5 µm) is shown in the lower left corner of the WT panel. (E) Kinetics of in vivo ferric chloride-induced thrombosis of mesenteric vessels. Venules (v) or arterioles (a) are shown by fluorescence microscopy (limits outlined with white dashed lines), thrombi being visualized by rhodamine 6G–labeled platelets. Images at 0, 30, and 60 minutes are shown. (F) Quantification of thrombus formation. Time to occlusion was noted for 18 control (WT, closed circles) and 21 SERCA3−/− (open circles) mice up to 60 minutes, the maximal time assessed. Results were analyzed using 1-way ANOVA followed by Tukey’s multiple comparison test; ***P < .001. (G) Quantification of emboli. The number of emboli shedding from thrombi was assessed for 60 minutes, both in venules and arterioles of control and SERCA3−/− mice.
Ferric chloride-induction of thrombosis in mesenteric vessels showed delayed thrombus formation (often no occlusion at 60 minutes) in both venules and arterioles of SERCA3−/− mice compared with controls (30 minutes occlusion time for controls; Figure 1E-F). Thrombus instability in SERCA3−/− mice was frequent with 3 times more venule or arteriole emboli than in controls (Figure 1G). These results indicate that SERCA3 ablation affects hemostasis, thrombus formation, and stability in vivo.
Evidence for an ADP secretion defect of SERCA3−/− platelets in in vitro flow adhesion and aggregation
To confirm a platelet defect, we next assessed adhesion and thrombus formation of SERCA3−/− platelets on a collagen matrix in both low (150 s−1; Figure 2A-B) and high (1200 s−1; Figure 2C-D) shear conditions, which were both significantly lower in SERCA3−/− platelets compared with controls. Adhesion and thrombus size (in both low and high shear conditions) of control platelets was diminished to the level of SERCA3−/− platelets after secretion inhibition by apyrase and indomethacin. Altogether these results indicate that SERCA3 ablation affected platelets, most likely through alteration of ADP secretion.
In vitro thrombus formation of SERCA3−/− platelets compared with controls. Rhodamine 6G–labeled platelets were injected into capillaries precoated with collagen (50 μg/mL) at low (150 s−1; A) or at high (1200 s−1; C) shear rates in absence or in presence of both apyrase (5 U/mL) and indomethacin (5 µM), noted “Apy-Indo.” Images show platelet adhesion and thrombus formation after 3 minutes of perfusion, and plots (B, D) represent the quantification of platelet adhesion, expressed as covered surface area relative to controls given as 100%. Absence or presence of Apy-Indo is noted “-” or “+” below plots. Data were calculated using 1-way ANOVA followed by Tukey’s multiple comparison test; ns, not significant; ***P < .001.
In vitro thrombus formation of SERCA3−/− platelets compared with controls. Rhodamine 6G–labeled platelets were injected into capillaries precoated with collagen (50 μg/mL) at low (150 s−1; A) or at high (1200 s−1; C) shear rates in absence or in presence of both apyrase (5 U/mL) and indomethacin (5 µM), noted “Apy-Indo.” Images show platelet adhesion and thrombus formation after 3 minutes of perfusion, and plots (B, D) represent the quantification of platelet adhesion, expressed as covered surface area relative to controls given as 100%. Absence or presence of Apy-Indo is noted “-” or “+” below plots. Data were calculated using 1-way ANOVA followed by Tukey’s multiple comparison test; ns, not significant; ***P < .001.
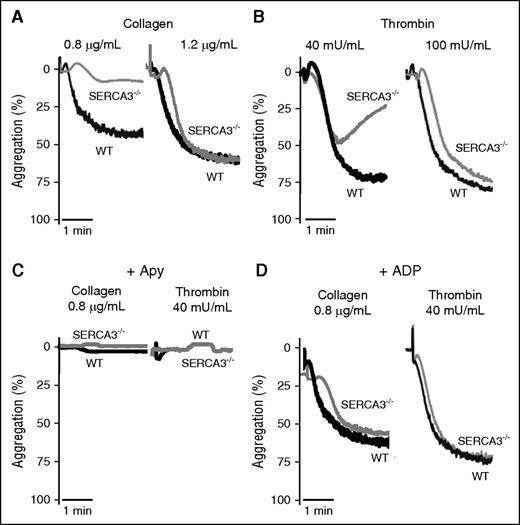
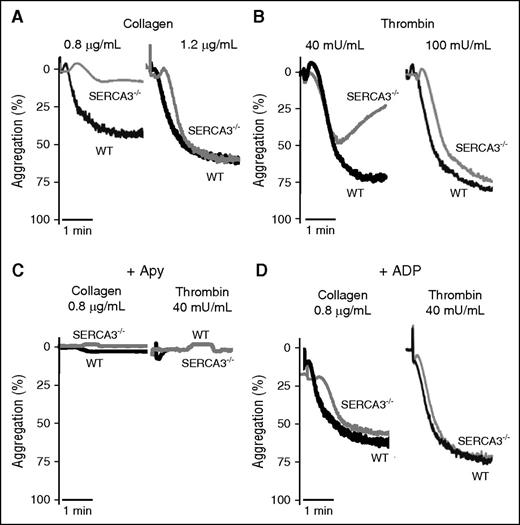
Confirming platelet involvement, aggregation of SERCA3−/− platelets was impaired, when induced by low levels of collagen (0.8 µg/mL) (Figure 3A), thrombin (40 mU/mL) (Figure 3B), or PAR4-AP peptide (activator peptide of the PAR-4 thrombin receptor, a G-protein coupled receptor) (supplemental Figure 2A). Higher doses of agonist essentially normalized aggregation levels. ADP scavenging by apyrase abolished aggregation responses of both control and SERCA3−/− platelets (Figure 3C; supplemental Figure 2B). This strengthened the hypothesis of an ADP secretion defect in SERCA3−/− platelets. Moreover aggregation of SERCA3−/− platelets was rescued by 10 µM ADP (not promoting aggregation alone in absence of added fibrinogen, not shown) addition to either collagen or thrombin (Figure 3D).
Aggregation of washed platelets from control or SERCA3−/− mice. Washed platelets from controls (WT, black line) or SERCA3−/− (gray line) mice were stimulated with collagen (A; 0.8 or 1.2 µg/mL) or with thrombin (B; 40 and 100 mU/mL) and recorded for aggregation for 3 minutes. Aggregation intensities are expressed as percent of light transmitted, 100% corresponding to buffer alone. Note the low aggregation rate of SERCA3−/− platelets at 0.8 µg/mL of collagen and 40 mU/mL thrombin. These tracings are representative of 5 experiments. (C) Aggregation induced by collagen (0.8 µg/mL) or thrombin (40 mU/mL) of control (WT) and SERCA3−/− platelets was carried out in the presence of apyrase (5 U/mL, noted “Apy”). (D) Aggregation rescue was conducted on control and SERCA3−/− washed platelets by addition of 10 µM ADP following stimulation by either collagen (0.8 µg/mL) or thrombin (40 mU/mL). These tracings are representative of 3 experiments.
Aggregation of washed platelets from control or SERCA3−/− mice. Washed platelets from controls (WT, black line) or SERCA3−/− (gray line) mice were stimulated with collagen (A; 0.8 or 1.2 µg/mL) or with thrombin (B; 40 and 100 mU/mL) and recorded for aggregation for 3 minutes. Aggregation intensities are expressed as percent of light transmitted, 100% corresponding to buffer alone. Note the low aggregation rate of SERCA3−/− platelets at 0.8 µg/mL of collagen and 40 mU/mL thrombin. These tracings are representative of 5 experiments. (C) Aggregation induced by collagen (0.8 µg/mL) or thrombin (40 mU/mL) of control (WT) and SERCA3−/− platelets was carried out in the presence of apyrase (5 U/mL, noted “Apy”). (D) Aggregation rescue was conducted on control and SERCA3−/− washed platelets by addition of 10 µM ADP following stimulation by either collagen (0.8 µg/mL) or thrombin (40 mU/mL). These tracings are representative of 3 experiments.
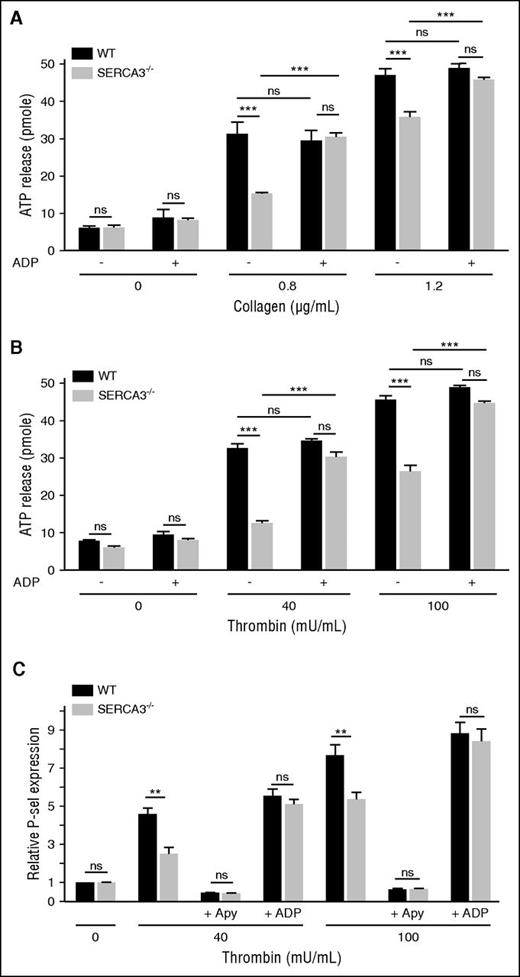
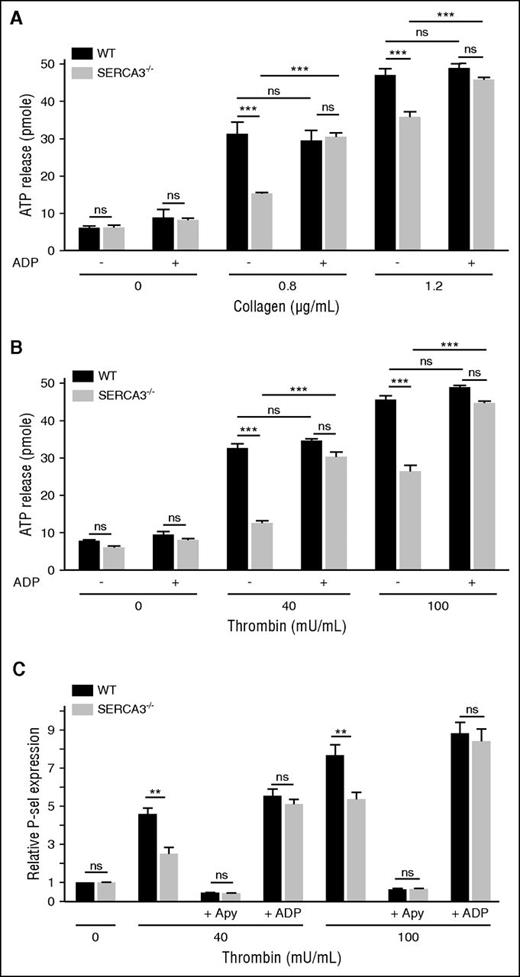
Secretion of the ADP storage organelles dense granules, as monitored by ATP release during aggregation appeared strongly diminished in SERCA3−/− compared with control platelets (Figure 4A-B; supplemental Figure 2C). Confirming a dense granule secretion defect, serotonin (5HT) release was also markedly altered in SERCA3−/− platelets (supplemental Figure 3C). Conversely, dense granule secretion was almost completely rescued by addition of 10 µM ADP, as assessed by ATP or 5HT secretion (Figure 4A; supplemental Figure 3C, respectively). ADP alone (Figure 4A-B) did not elicit dense granule secretion in control or SERCA3−/− platelets. Thus, SERCA3−/− platelets exhibit a defect because of alteration of dense granule secretion.
Platelet secretion of washed platelets from control or SERCA3−/− mice. Dense granule secretion from platelets aggregated in the presence of collagen (A; 0.8 or 1.2 µg/mL) or thrombin (B; 40 or 100 mU/mL), with the addition (+) or not (-) of 10 µM ADP, was assessed by measuring ATP release in picomoles (calculated for 107 platelets) in control (WT, black bars) and SERCA3−/− (gray bars) platelets. A total of 3 experiments were conducted and presented as mean ± SEM, using 1-way ANOVA followed by Tukey’s multiple comparison test; ns, not significant; ***P < .001. (C) The expression of the α-granule membrane marker P-selectin following thrombin platelet stimulation was assessed by flow cytometry on control (black bars) or SERCA3−/− (gray bars) platelets. The same experiment was conducted in the presence of apyrase (5 U/mL, noted “+ Apy”) or added ADP (10 µM, noted “+ ADP”) on control (WT, black bars) or SERCA3−/− (gray bars) platelets. Data presented are the means of 3 separate experiments in duplicates, as means ± SEM, as assessed by 1-way ANOVA followed by Tukey’s multiple comparison test; ns, not significant; **P < .01.
Platelet secretion of washed platelets from control or SERCA3−/− mice. Dense granule secretion from platelets aggregated in the presence of collagen (A; 0.8 or 1.2 µg/mL) or thrombin (B; 40 or 100 mU/mL), with the addition (+) or not (-) of 10 µM ADP, was assessed by measuring ATP release in picomoles (calculated for 107 platelets) in control (WT, black bars) and SERCA3−/− (gray bars) platelets. A total of 3 experiments were conducted and presented as mean ± SEM, using 1-way ANOVA followed by Tukey’s multiple comparison test; ns, not significant; ***P < .001. (C) The expression of the α-granule membrane marker P-selectin following thrombin platelet stimulation was assessed by flow cytometry on control (black bars) or SERCA3−/− (gray bars) platelets. The same experiment was conducted in the presence of apyrase (5 U/mL, noted “+ Apy”) or added ADP (10 µM, noted “+ ADP”) on control (WT, black bars) or SERCA3−/− (gray bars) platelets. Data presented are the means of 3 separate experiments in duplicates, as means ± SEM, as assessed by 1-way ANOVA followed by Tukey’s multiple comparison test; ns, not significant; **P < .01.
P-selectin exposure of stimulated SERCA3−/− platelets (Figure 4C; supplemental Figure 2E) was significantly diminished compared with control platelets. Not shown, a defect in P-selectin exposure was also observed in convulxin-stimulated SERCA3−/− unstirred platelets. Apyrase treatment nearly suppressed P-selectin exposure of control and SERCA3−/− platelets, stimulated with low thrombin concentration, whereas ADP addition to thrombin restored normal P-selectin exposure on SERCA3−/− platelets (Figure 4C). This indicated that α-granule exocytosis is likely to be secondary to ADP secretion and thus only indirectly dependent on SERCA3.
Finally, aggregation to ADP of SERCA3−/− platelets (in platelet-rich plasma, to provide fibrinogen), was normal compared with control (Figure 5C). Thus, SERCA3−/− ablation does not affect platelet activation by ADP but specifically acts on ADP secretion.
Assessment of αIIb β3 activation and engagement in washed platelets from control or SERCA3−/− mice and aggregation to ADP. (A) Quantitation of activated αIIbβ3 integrin at the surface of washed platelets was assessed by flow cytometry by binding of the specific mAb JON/A to control (black bars) or SERCA3−/− (gray bars) platelets upon activation with thrombin at 40 or 100 mU/mL. The same experiments were conducted in the presence of 5 U/mL apyrase (noted “+ Apy”) or after addition of 10 µM ADP (“+ ADP”). Statistical significance was established with 1-way ANOVA followed by Tukey’s multiple comparison test; ns, not significant; **P < .01. (B) Assessment of the role of αIIbβ3 engagement in dense granule secretion in SERCA3−/− platelets. Platelets stimulated with either 40 or 100 mU/mL of thrombin were subjected to aggregation, in the absence (-) or the presence (+) of the blocking mAb Leo.H4 (20 µg/mL) specific for mouse αIIbβ3, as well as in the absence (-) or the presence (+) of 10 µM ADP. Secretion was assessed by ATP measurement in the supernatant. Using WT as control, statistical significance was established with 1-way ANOVA followed by Tukey’s multiple comparison test; ns, not significant; **P < .01; ***P < .001. (C) Aggregation to ADP was assessed at 0.25, 0.5, 1, 2.5, 5, and 10 µM in control (WT) or SERCA3−/− platelet-rich plasma.
Assessment of αIIb β3 activation and engagement in washed platelets from control or SERCA3−/− mice and aggregation to ADP. (A) Quantitation of activated αIIbβ3 integrin at the surface of washed platelets was assessed by flow cytometry by binding of the specific mAb JON/A to control (black bars) or SERCA3−/− (gray bars) platelets upon activation with thrombin at 40 or 100 mU/mL. The same experiments were conducted in the presence of 5 U/mL apyrase (noted “+ Apy”) or after addition of 10 µM ADP (“+ ADP”). Statistical significance was established with 1-way ANOVA followed by Tukey’s multiple comparison test; ns, not significant; **P < .01. (B) Assessment of the role of αIIbβ3 engagement in dense granule secretion in SERCA3−/− platelets. Platelets stimulated with either 40 or 100 mU/mL of thrombin were subjected to aggregation, in the absence (-) or the presence (+) of the blocking mAb Leo.H4 (20 µg/mL) specific for mouse αIIbβ3, as well as in the absence (-) or the presence (+) of 10 µM ADP. Secretion was assessed by ATP measurement in the supernatant. Using WT as control, statistical significance was established with 1-way ANOVA followed by Tukey’s multiple comparison test; ns, not significant; **P < .01; ***P < .001. (C) Aggregation to ADP was assessed at 0.25, 0.5, 1, 2.5, 5, and 10 µM in control (WT) or SERCA3−/− platelet-rich plasma.
Dense granule content is not affected in SERCA3−/− platelets
To check that defective secretion was not because of a dense granule defect, platelets were labeled with the fluorescent reporter mepacrine, which accumulates specifically in dense granules.31 Flow cytometry showed that mepacrine was stored to the same extent in control and SERCA3−/− platelets (supplemental Figure 3A), strongly suggesting a normal content in dense granules. In addition ATP release after maximal platelet stimulation (thrombin 2 U/mL) of SERCA3−/− platelets reached ∼70% of controls (supplemental Figure 3B), but reached 100% ATP secretion compared with control platelets upon ADP addition to thrombin. Most significantly, total 5HT content was identical between control and SERCA3−/− platelets (supplemental Figure 3C).
Thus, the dense granule secretion defect observed in SERCA3−/− platelets is not because of a defect in number or content.
5HT does not restore the functional defect of SERCA3−/− platelets
To test whether rescue is specific for ADP, and may not be induced just by any weak agonist, we analyzed aggregation and secretion rescue induced by 5HT (another weak agonist and dense granule cargo). At concentrations within the same range as ADP, 5HT did not induce aggregation (supplemental Figure 3D), nor did it rescue aggregation (supplemental Figure 3E), ATP secretion (supplemental Figure 3F), αIIbβ3 activation, or P-selectin exposure (data not shown) elicited by 40 mU/mL thrombin. These data, together with the defects induced by apyrase (flow adhesion, platelet aggregation, and secretion) in control platelets, confirm that ADP is most likely the only agonist involved in SERCA3−/− platelet defect.
SERCA3 ablation alters ADP-dependent αIIbβ3 activation
Platelet secretion is elicited by agonist-induced receptor activation and αIIbβ3 integrin engagement (outside-in signaling). To examine a potential link between SERCA3-dependent ADP secretion and αIIbβ3, SERCA3−/− platelets were activated by thrombin in the presence of the mAb JON/A, specific for the active form of mouse αIIbβ3,32 and analyzed by flow cytometry. SERCA3−/− platelets exhibited lower αIIbβ3 activation [but normal total level (supplemental Figure 1)] than controls (Figure 5A). ADP scavenging by apyrase almost completely abrogated αIIbβ3 activation in both control and SERCA3−/− platelets (Figure 5A). Conversely, addition of ADP rescued αIIbβ3 activation in both cases, confirming that the lower activation level of αIIbβ3 in SERCA3−/− platelets is because of reduced ADP secretion. Blockade of αIIbβ3 by the antibody Leo.H4,33 which prevents αIIbβ3 engagement, reduced secretion equally in both control and SERCA3−/− platelets, corresponding to αIIbβ3-dependent secretion, but the difference in secretion remained unchanged (Figure 5B). Importantly, ADP addition rescued secretion in SERCA3−/− platelets (unchanged in control platelets) despite αIIbβ3 blockade. Thus, the altered secretion in SERCA3−/− platelets is αIIbβ3 independent.
SERCA3 ablation or inhibition alters Ca2+ signaling and dense granule secretion
SERCA3 and SERCA2b34 regulate Ca2+ mobilization from intracellular stores, essential to platelet activation and secretion.35 Flow cytometry of unstirred Oregon Green BAPTA1-AM loaded platelets preincubated with EGTA (no extracellular Ca2+ to avoid Ca2+ influx) and stimulated with thrombin showed a lower Ca2+ mobilization (50%, as measured by the area below the curve for 2 minutes) in SERCA3−/− platelets compared with controls, at 40 mU/mL (Figure 6A,E, left panels), but not at 100 mU/mL (supplemental Figure 4A). Ca2+ influx induced by extracellular Ca2+ (1 mM) was stronger in SERCA3−/− (30% to 40% increase as assessed by areas under curves) than control platelets (Figure 6A-B,E; supplemental Figure 4A). Interestingly, stimulation of SERCA3−/− platelets with thrombin in the presence of extracellular Ca2+ showed a similar fluorescence increase compared with control platelets, suggesting compensation of low Ca2+ mobilization by high Ca2+ influx (Figure 6A, right panel). Importantly, in the presence of apyrase, Ca2+ mobilization of control platelets at 40 mU/mL of thrombin was lowered to the level of SERCA3−/− platelets, unaffected by ADP scavenging (Figure 6B,E). Conversely, addition of ADP to thrombin raised Ca2+ mobilization in SERCA3−/− platelets to the level of control platelets (Figure 6B,E). In contrast, Ca2+ influx remained unaffected by apyrase pretreatment or after addition of ADP, for both control and SERCA3−/− platelets (Figure 6B,E), suggesting that contrary to mobilization, influx is independent of ADP. These results are thus consistent with SERCA3-dependent Ca2+ mobilization in low agonist conditions being secondary to secreted ADP.
Effect of SERCA3 deletion or pharmacological inhibition on Ca2+ mobilization, Ca2+ influx, aggregation, and secretion in washed platelets. (A) Ca2+ mobilization was assessed in unstirred control (WT, black tracings) and SERCA3−/− (gray tracings) platelets preincubated with the cytosolic Ca2+ fluorescent probe Oregon Green BAPTA-AM after stimulation with 40 mU/mL thrombin (“Thr”) by flow cytometry in conditions of no external Ca2+ (1 mM EGTA). Ca2+ influx was assessed after 4 minutes by addition of 1 mM CaCl2 (“Ca2+”). Global Ca2+ signaling was also assessed by addition of 1 mM Ca2+ together with thrombin (“Thr + Ca2+”) (right tracing). Data are expressed as nM Ca2+, as calculated from calibration experiments (see supplemental Methods). (B) Ca2+ mobilization was assessed in the same conditions as in panel A, but in the presence of apyrase (5 U/mL) (“+ Apy”) or ADP (1 µM) (“+ ADP”). (C) Ca2+ mobilization by 40 mU/mL thrombin was assessed after preincubation with the SERCA3-specific inhibitor tBHQ (C; 10 µM) or with Tg (D) at a concentration affecting only SERCA2b (200 nM; see supplemental Figure 5A). (E) Maximal Ca2+ mobilization and Ca2+ influx from experiments in panel A (stimulation with 40 mU/mL thrombin in the presence of 100 µM EGTA and Ca2+ influx after CaCl2 [300 µM] addition) and in panel B (same as in panel A, but in the presence of 5 U/mL apyrase [Thr + Apy] or of 10 µM ADP [Thr + ADP]). Values were calculated after subtraction of unstimulated Ca2+ level (ΔCa2+ nM). Data presented are means ± SEM, n = 3, using 1-way ANOVA followed by Tukey’s multiple comparison test: ns, not significant; **P < .01; ***P < .001. (F) Washed control platelets were preincubated with dimethyl sulfoxide (control) or tBHQ 10 µM (tBHQ) or tBHQ and ADP (10 µM each) for 4 minutes prior to addition of 40 mU/mL thrombin (Thr). (G) Washed control (WT, black bars) or SERCA3−/− (gray bars) platelets were preincubated with either buffer alone “-”, tBHQ (10 µM), or Tg (200 nM), and then either buffer (“0”), thrombin 40 mU/mL, or thrombin and 10 µM ADP (“+ ADP”) and incubated further for 3 minutes. ATP secretion was then measured in supernatants. Data presented are means ± SEM, n = 3, using 1-way ANOVA followed by Tukey’s multiple comparison test: ns, not significant; ***P < .001.
Effect of SERCA3 deletion or pharmacological inhibition on Ca2+ mobilization, Ca2+ influx, aggregation, and secretion in washed platelets. (A) Ca2+ mobilization was assessed in unstirred control (WT, black tracings) and SERCA3−/− (gray tracings) platelets preincubated with the cytosolic Ca2+ fluorescent probe Oregon Green BAPTA-AM after stimulation with 40 mU/mL thrombin (“Thr”) by flow cytometry in conditions of no external Ca2+ (1 mM EGTA). Ca2+ influx was assessed after 4 minutes by addition of 1 mM CaCl2 (“Ca2+”). Global Ca2+ signaling was also assessed by addition of 1 mM Ca2+ together with thrombin (“Thr + Ca2+”) (right tracing). Data are expressed as nM Ca2+, as calculated from calibration experiments (see supplemental Methods). (B) Ca2+ mobilization was assessed in the same conditions as in panel A, but in the presence of apyrase (5 U/mL) (“+ Apy”) or ADP (1 µM) (“+ ADP”). (C) Ca2+ mobilization by 40 mU/mL thrombin was assessed after preincubation with the SERCA3-specific inhibitor tBHQ (C; 10 µM) or with Tg (D) at a concentration affecting only SERCA2b (200 nM; see supplemental Figure 5A). (E) Maximal Ca2+ mobilization and Ca2+ influx from experiments in panel A (stimulation with 40 mU/mL thrombin in the presence of 100 µM EGTA and Ca2+ influx after CaCl2 [300 µM] addition) and in panel B (same as in panel A, but in the presence of 5 U/mL apyrase [Thr + Apy] or of 10 µM ADP [Thr + ADP]). Values were calculated after subtraction of unstimulated Ca2+ level (ΔCa2+ nM). Data presented are means ± SEM, n = 3, using 1-way ANOVA followed by Tukey’s multiple comparison test: ns, not significant; **P < .01; ***P < .001. (F) Washed control platelets were preincubated with dimethyl sulfoxide (control) or tBHQ 10 µM (tBHQ) or tBHQ and ADP (10 µM each) for 4 minutes prior to addition of 40 mU/mL thrombin (Thr). (G) Washed control (WT, black bars) or SERCA3−/− (gray bars) platelets were preincubated with either buffer alone “-”, tBHQ (10 µM), or Tg (200 nM), and then either buffer (“0”), thrombin 40 mU/mL, or thrombin and 10 µM ADP (“+ ADP”) and incubated further for 3 minutes. ATP secretion was then measured in supernatants. Data presented are means ± SEM, n = 3, using 1-way ANOVA followed by Tukey’s multiple comparison test: ns, not significant; ***P < .001.
To confirm the role of SERCA3 and its catalytic activity, platelets were challenged with tBHQ specific for SERCA3.3 In control platelets, 10 µM tBHQ (specific for SERCA3, because inducing Ca2+ mobilization in control but not in SERCA3−/− platelets; supplemental Figure 4C) lowered thrombin-induced Ca2+ mobilization (Figure 6C) comparatively to control (Figure 6A). Ratios of areas under curves of WT over SERCA3−/− Ca2+ mobilization dropped from 2 in absence of tBHQ to 1 in the presence of the antagonist. tBHQ elicited partial inhibition of thrombin-induced aggregation (Figure 6F) and secretion (Figure 6G) of SERCA3−/− platelets, which were both rescued by ADP. In contrast, specific inhibition of SERCA2b with 200 nM Tg (supplemental Figure 5A) partially inhibited thrombin-induced Ca2+ mobilization in control platelets (presumably leaving SERCA3-dependent Ca2+ stores unaffected) but completely inhibited mobilization in SERCA3−/− platelets, indicating complete inhibition of SERCA2b (Figure 6D). Importantly, specific inhibition of SERCA2b did not affect ATP release, contrary to tBHQ-mediated SERCA3 inhibition (Figure 6G). Altogether these results thus demonstrate that catalytically active SERCA3, and not SERCA2b, is involved in dense granule secretion, through its Ca2+ pump activity and SERCA3-dependent Ca2+ stores.
Ca2+ stores are defective in SERCA3−/− platelets
SERCA3 depletion is expected to lead to depletion of SERCA3-dependent Ca2+ stores and hence to altered Ca2+ mobilization. Platelets were subjected to ionomycin, which permeabilizes inner membranes and empties Ca2+ stores, and to Tg at high concentration (1 µM) to prevent Ca2+ store reuptake by both SERCA3 and SERCA2b (supplemental Figure 4B). SERCA3−/− platelets exhibited a weaker cytosolic Ca2+ release signal than control platelets (0.65 relative ratio assessed by areas under the curves) consistent with partially depleted Ca2+ stores in SERCA3−/− platelets. Conversely, Ca2+ reuptake was assessed in control and SERCA3−/− platelets by Ca2+ mobilization triggered by thrombin, followed after 3 minutes of stimulation (allowing Ca2+ store refilling) by Tg (1 µM, inhibiting both SERCAs) to let stored Ca2+ “leak” into the cytosol (supplemental Figure 5C). Tg triggered significantly less Ca2+ release in SERCA3−/− platelets than in control platelets, consistent with less efficient Ca2+ reuptake in SERCA3−/− platelets than in controls, underlining the functional relevance of SERCA3. Both the lower levels of Ca2+ store release and the low Ca2+ store reuptake in SERCA3−/− platelets strongly suggest that SERCA3-dependent Ca2+ stores are involved, at least in part, in dense granule secretion.
Discussion
We have analyzed SERCA3−/− mice and found a significant prolonged bleeding time and defective thrombosis in vivo. Functional assessment of SERCA3−/− platelets in vitro has confirmed a defect in adhesion, thrombus formation over collagen, and aggregation elicited by either collagen or thrombin. This indicated that platelets were directly affected by SERCA3 ablation, and that the defect was independent of the stimulus pathway. Most importantly, we found that the defect could be tracked down to a markedly reduced secretion as assessed by ATP measurement and, thus, presumably reduced dense granule exocytosis. Importantly all affected functions in platelet SERCA3−/−, including adhesion under flow, aggregation, and secretion, were reversed by exogenous ADP, adding more support to the idea that ablation of SERCA3 leads to an ADP secretion defect. Experiments conducted with 5HT showed that (1) this weak agonist also stored in dense granules is defective to the same extent as ATP, confirming that the defect lies in the release mechanism of dense granules; (2) like ATP, it reaches normal secretion when ADP is added to thrombin; but (3) unlike ADP, it is unable to restore normal aggregation or secretion of SERCA3−/− platelets upon thrombin stimulation. This strengthens the hypothesis that SERCA3 depletion does affect release of a fraction of dense granules, and that this release is specifically dependent upon ADP costimulation, and not costimulation by just any weak agonist.
In addition, this SERCA3-dependent secretion is independent of αIIbβ3 engagement and only dependent on primary platelet activation. Importantly, aggregation to ADP of SERCA3−/− platelets was normal indicating that ADP-dependent activation pathways of platelets were not altered by SERCA3 ablation. Our results indicate also that SERCA3 ablation does not affect dense granules, as evidenced by normal mepacrine content, normal serotonin content, or electron microscopy imaging. It follows that SERCA3 ablation is responsible for alteration of dense granule secretory rather than storage pathways.
Importantly, pharmacological inhibition of SERCA3 but not of SERCA2b recapitulated the ADP secretory defect of SERCA3 genetic ablation, clearly showing a specific role for SERCA3 in secretion. Moreover, this demonstrates that SERCA3 catalytic activity is required for secretion, pointing to SERCA3-dependent Ca2+ storage and/or signaling as involved in secretion regulation. Of note, when platelets were maximally stimulated with 2 U/mL thrombin, SERCA3−/− platelets still exhibited a significant differential secretion compared with controls, whether assessed by ATP or 5HT release (supplemental Figure 3B-C, respectively), only compensated for by added ADP. This secretion resistance to strong stimulation argues in favor of a pool of dense granules present but not releasable in SERCA3-deleted platelets. This would thus be consistent with 2 physically and/or functionally separate ADP secretory pathways, possibly corresponding to distinct populations of dense granules and/or to different exocytosis pathways, 1 involving SERCA3 (and its Ca2+ stores) and not the other. An attractive hypothesis could be that this subpopulation of dense granules corresponds to SERCA3-dependent Ca2+-stores, explaining the link between ADP release and SERCA3. However, we found no colocalization between SERCA3 and dense granules by confocal microscopy (data not shown): we conclude that SERCA3-dependent Ca2+ stores are distinct from dense granules but regulate exocytosis of a subpopulation of dense granules, for example proximal to the plasma membrane, allowing early release of ADP.
We noted that α-granule secretion, in absence of αIIbβ3 engagement, is almost completely dependent on ADP release. This is consistent with an earlier report showing that dense granules are mobilized earlier than α-granules36 and with 2 recent reports showing that mouse models of Hermansky-Pudlak syndrome, which are defective in dense granules, exhibit, upon low agonist stimulation or laser-induced injury in vivo, a defect in α-granule (and lysosome) secretion secondary to the lack of ADP secretion.37,38 Moreover, their observations of an autocrine ADP secretion39 are consistent with our contention that ADP secretion (here SERCA3-dependent) reinforces platelet activation by other agonists.
Interestingly, there was an increased level of SOCE in SERCA3−/− platelets, apparently compensating for the low level of Ca2+ mobilization (such that the overall Ca2+ cytosolic rise upon agonist stimulation in the presence of external Ca2+ was normal). This compensating SOCE is consistent with the low levels of Ca2+ stores in SERCA3−/− platelets, known to induce recruitment of STIM1 and STIM2 by Orai-1.40 Of note, this sustained SOCE is not modulated by ADP scavenging or addition, strengthening the idea that it is only driven by Ca2+ store depletion, and not via an ADP-dependent pathway. Thus, SOCE appears as a compensatory mechanism in the context of SERCA3 ablation (or inhibition), possibly explaining the limited hemostasis impact on SERCA3−/− mice. Interestingly, Harper et al have shown that NC(K)X Ca2+ exchangers drive initial SOCE upon Tg-induced Ca2+ stores depletion, triggering dense granule secretion, which potentiated activation through ADP, ATP, and 5HT pathways.41 Although this observation points to a link between Ca2+ regulation and an autocrine platelet activation amplification, it clearly acts in a different manner because it involves NC(K)X and SOCE, not being specific for ADP.
Importantly, tBHQ-mediated SERCA3 inhibition in human platelets leads to defective aggregation, ATP secretion, and αIIbβ3 activation (Z.E., R.B., M.B., and J.-P.R., unpublished data, December 20, 2015). Thus, SERCA3 plays the same role in human and mouse platelets.
Finally, SERCA3 depletion or inhibition appears to lead to defective SERCA3-dependent Ca2+ storing. This conclusion stems from several convergent observations: First, mobilization of Ca2+ from intracellular compartments was clearly affected in SERCA3−/− platelets, independent of the agonist used. Second, SERCA3−/− platelets exhibit stored Ca2+ levels lower than control platelets, as evidenced by experiments assessing release of total Ca2+ stores by ionomycin and Tg (supplemental Figure 4B). Third, blockade by the SERCA3-specific pharmacological inhibitor tBHQ (leading to Ca2+ “leakage” from SERCA3-dependent Ca2+ stores), in conditions (10 µM) where it did elicit Ca2+ release in the cytosol of control but not of SERCA3−/− platelets, reproduced the defect in Ca2+ mobilization, as well as in secretion. This effect was SERCA3-specific because it was not observed with 200 nM Tg, specific for SERCA2b. One can thus conclude that a Ca2+ storage pool dependent on SERCA3, and not on SERCA2b, seems to be required for a release of ADP, itself seemingly important to full platelet activation in conditions of low agonist concentration.
In conclusion, our data provide evidence that platelet activation seems to involve a release of ADP through a secretory pathway under the control of SERCA3-dependent Ca2+ stores, independently from αIIbβ3 integrin engagement. The link between SERCA3-dependent Ca2+ stores and SERCA3-dependent ADP stores remains to be established.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank P. Gilon and G. E. Shull for providing the SERCA3−/− mice; Emilie Namur for setting up the Oregon Green fluorescence-Ca2+ standard curves; and Christelle Repérant for help with platelet preparation and flow cytometry. The authors also thank the CeCILE-SFR/Centre Commun d’Imagerie de Lyon-Est–Structure Fédérative de Recherche (France) for expert technical assistance in electron microscopy studies.
Z.E. is a PhD candidate at Université Paris-Sud, and this work is submitted in partial fulfillment of the requirement for a PhD.
This work was supported in part by INSERM and Université Paris-Sud, as well as a fellowship from the Ministère de l’Education Nationale, de l’Enseignement Supérieur et de la Recherche (Z.E.).
Authorship
Contribution: Z.E., F.A., E.B., J.-C.B., R.B., and M.B. performed experiments; Z.E., R.B., M.B., and J.-P.R. analyzed results; R.B., F.A., M.B., and J.-P.R. designed experiments; J.-P.R. wrote the manuscript; and Z.E., F.A., N.P., R.B., M.B., and J.-P.R. critically edited the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Jean-Philippe Rosa, INSERM U1176, Hôpital Bicêtre, 82 rue du Général Leclerc, 94276 Le Kremlin Bicêtre Cedex, France; e-mail: jean-philippe.rosa@inserm.fr.
References
Author notes
R.B., M.B., and J.-P.R. contributed equally to this study.



![Figure 6. Effect of SERCA3 deletion or pharmacological inhibition on Ca2+ mobilization, Ca2+ influx, aggregation, and secretion in washed platelets. (A) Ca2+ mobilization was assessed in unstirred control (WT, black tracings) and SERCA3−/− (gray tracings) platelets preincubated with the cytosolic Ca2+ fluorescent probe Oregon Green BAPTA-AM after stimulation with 40 mU/mL thrombin (“Thr”) by flow cytometry in conditions of no external Ca2+ (1 mM EGTA). Ca2+ influx was assessed after 4 minutes by addition of 1 mM CaCl2 (“Ca2+”). Global Ca2+ signaling was also assessed by addition of 1 mM Ca2+ together with thrombin (“Thr + Ca2+”) (right tracing). Data are expressed as nM Ca2+, as calculated from calibration experiments (see supplemental Methods). (B) Ca2+ mobilization was assessed in the same conditions as in panel A, but in the presence of apyrase (5 U/mL) (“+ Apy”) or ADP (1 µM) (“+ ADP”). (C) Ca2+ mobilization by 40 mU/mL thrombin was assessed after preincubation with the SERCA3-specific inhibitor tBHQ (C; 10 µM) or with Tg (D) at a concentration affecting only SERCA2b (200 nM; see supplemental Figure 5A). (E) Maximal Ca2+ mobilization and Ca2+ influx from experiments in panel A (stimulation with 40 mU/mL thrombin in the presence of 100 µM EGTA and Ca2+ influx after CaCl2 [300 µM] addition) and in panel B (same as in panel A, but in the presence of 5 U/mL apyrase [Thr + Apy] or of 10 µM ADP [Thr + ADP]). Values were calculated after subtraction of unstimulated Ca2+ level (ΔCa2+ nM). Data presented are means ± SEM, n = 3, using 1-way ANOVA followed by Tukey’s multiple comparison test: ns, not significant; **P < .01; ***P < .001. (F) Washed control platelets were preincubated with dimethyl sulfoxide (control) or tBHQ 10 µM (tBHQ) or tBHQ and ADP (10 µM each) for 4 minutes prior to addition of 40 mU/mL thrombin (Thr). (G) Washed control (WT, black bars) or SERCA3−/− (gray bars) platelets were preincubated with either buffer alone “-”, tBHQ (10 µM), or Tg (200 nM), and then either buffer (“0”), thrombin 40 mU/mL, or thrombin and 10 µM ADP (“+ ADP”) and incubated further for 3 minutes. ATP secretion was then measured in supernatants. Data presented are means ± SEM, n = 3, using 1-way ANOVA followed by Tukey’s multiple comparison test: ns, not significant; ***P < .001.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/128/8/10.1182_blood-2015-10-678383/4/m_1129f6.jpeg?Expires=1770247192&Signature=T1yZxzkLkgosFxJdn9106mCvUxGUxIFerAzH7rjPxG6yYA94ih15FNAgR2YiYWjtNMhsaN2kNaZ~ACSlm2ezXcEbkYUH13uIeWX2LeS7-xrQ8vhGS3jtzz-AqfKqQjX~cXmDLjvDdIXKs2klMhHbbgCVZNEeFasJX1SdtY9Eu~dDhmLWqqaJxbyi6UcF7kqGZp9qgZqn4-fpkL-Iq84cSFz6QKJQcqDGREbaN6udHzZHfJ~HiJKMqhfukrSFw5QuIgxefaXlRWaYGNjFlvEEjgrMJd004R5xUnU~jMj3Ks1OMkGHV3J161KCgK-D7SResnnxPUIv9cLjqFYHpYTp2w__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)




![Figure 6. Effect of SERCA3 deletion or pharmacological inhibition on Ca2+ mobilization, Ca2+ influx, aggregation, and secretion in washed platelets. (A) Ca2+ mobilization was assessed in unstirred control (WT, black tracings) and SERCA3−/− (gray tracings) platelets preincubated with the cytosolic Ca2+ fluorescent probe Oregon Green BAPTA-AM after stimulation with 40 mU/mL thrombin (“Thr”) by flow cytometry in conditions of no external Ca2+ (1 mM EGTA). Ca2+ influx was assessed after 4 minutes by addition of 1 mM CaCl2 (“Ca2+”). Global Ca2+ signaling was also assessed by addition of 1 mM Ca2+ together with thrombin (“Thr + Ca2+”) (right tracing). Data are expressed as nM Ca2+, as calculated from calibration experiments (see supplemental Methods). (B) Ca2+ mobilization was assessed in the same conditions as in panel A, but in the presence of apyrase (5 U/mL) (“+ Apy”) or ADP (1 µM) (“+ ADP”). (C) Ca2+ mobilization by 40 mU/mL thrombin was assessed after preincubation with the SERCA3-specific inhibitor tBHQ (C; 10 µM) or with Tg (D) at a concentration affecting only SERCA2b (200 nM; see supplemental Figure 5A). (E) Maximal Ca2+ mobilization and Ca2+ influx from experiments in panel A (stimulation with 40 mU/mL thrombin in the presence of 100 µM EGTA and Ca2+ influx after CaCl2 [300 µM] addition) and in panel B (same as in panel A, but in the presence of 5 U/mL apyrase [Thr + Apy] or of 10 µM ADP [Thr + ADP]). Values were calculated after subtraction of unstimulated Ca2+ level (ΔCa2+ nM). Data presented are means ± SEM, n = 3, using 1-way ANOVA followed by Tukey’s multiple comparison test: ns, not significant; **P < .01; ***P < .001. (F) Washed control platelets were preincubated with dimethyl sulfoxide (control) or tBHQ 10 µM (tBHQ) or tBHQ and ADP (10 µM each) for 4 minutes prior to addition of 40 mU/mL thrombin (Thr). (G) Washed control (WT, black bars) or SERCA3−/− (gray bars) platelets were preincubated with either buffer alone “-”, tBHQ (10 µM), or Tg (200 nM), and then either buffer (“0”), thrombin 40 mU/mL, or thrombin and 10 µM ADP (“+ ADP”) and incubated further for 3 minutes. ATP secretion was then measured in supernatants. Data presented are means ± SEM, n = 3, using 1-way ANOVA followed by Tukey’s multiple comparison test: ns, not significant; ***P < .001.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/128/8/10.1182_blood-2015-10-678383/4/m_1129f6.jpeg?Expires=1770308390&Signature=b518BslMXmIitR2bKCCJY2QXxsKFjU4eN8Q4Pj7fTO1Ng7mHN7v35~UNjcayv5pnhwt83SEO3Uoz7JtIx1cG2~~Gypt0s-F95pekOtMZSXxApThDzLo4tPbNq6HzYDP2nQmW4ZZjmBR-QaYDxsX9waX6Doq~uEt5mPTYH0DODT7G40idHHKnNU9eVWkO7JGnY-f5rA-4~bKyhd0TeIp5HEIWdY5EmLkjhl60wiRPUOxxR40i16VyoEhrreclCOljG6BLUMwDer4-gwQ8vq5~5rzLd9YxK~Gfymas-gQLMItENs0FkKh69hU~GRKD4cLibaM~1QWwd78Jgnkv0l3SVA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)