In this issue of Blood, Elaïb et al evaluate the role of sarco-endoplasmic reticulum calcium (Ca2+) adenosine triphosphatase (ATPase) 3 (SERCA3) in platelet function, and discover an unexpectedly strong link between SERCA3 activity and dense granule secretion.1
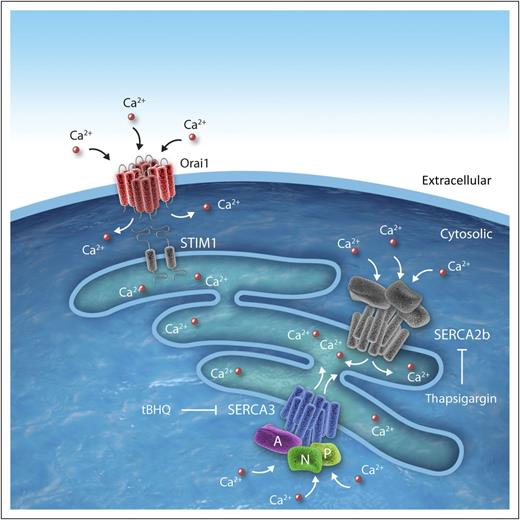
SERCA3 in platelets. SERCA3 is a multidomain calcium pump consisting of a transmembrane domains (blue), a phosphorylation domain (P, light green), a nucleotide-binding domain (N, green), and an actuator domain (A, purple). It functions as a calcium pump during SOCE in platelets. SOCE occurs when STIM1 detects depleted internal calcium stores (light blue) and stimulates entry of extracellular calcium into platelet cytosol via Orai1. SERCA3 and SERCA2b function to pump calcium into internal stores. tBHQ, 2,5-di-(tert-butyl)-1,4-benzohydroquinone. Professional illustration by Idoya Lahortiga, Somersault18:24.
SERCA3 in platelets. SERCA3 is a multidomain calcium pump consisting of a transmembrane domains (blue), a phosphorylation domain (P, light green), a nucleotide-binding domain (N, green), and an actuator domain (A, purple). It functions as a calcium pump during SOCE in platelets. SOCE occurs when STIM1 detects depleted internal calcium stores (light blue) and stimulates entry of extracellular calcium into platelet cytosol via Orai1. SERCA3 and SERCA2b function to pump calcium into internal stores. tBHQ, 2,5-di-(tert-butyl)-1,4-benzohydroquinone. Professional illustration by Idoya Lahortiga, Somersault18:24.
SERCAs are a family of calcium pumps. These P-type ATPases transport calcium into intracellular storage compartments in an energy-dependent process that requires cleavage of adenosine triphosphate (ATP) to adenosine 5′-diphosphate (ADP). The SERCA family is encoded by 3 genes, ATP2A1, ATP2A2, and ATP2A3, which produce multiple isoforms including SERCA1a/b, SERCA2a-c, and SERCA3a-f.2 Their domain structure includes the transmembrane domains, the phosphorylation domain, the nucleotide-binding domain, and the actuator domain. These domains are organized into a head (encompassing the phosphorylation, nucleotide-binding, and actuator domains) that resides in the cytoplasm, a stalk, and a transmembrane component (see figure).2
SERCA3 is perhaps the most enigmatic member of the family, distinguished on the basis of its wide tissue distribution and on being a nonmuscle calcium pump. It has a surprisingly low calcium affinity. For example, the affinity of SERCA2b for calcium is K1/2 ∼0.27 μM, whereas that of SERCA3 is K1/2 ∼1.1 μM.3 Platelets contain both SERCA2b and SERCA3. Platelet SERCA3 is distributed on intracellular membranes4 and has a known function in sequestering calcium into membrane compartments. Increased SERCA3 levels are associated with peripheral artery disease in the setting of diabetes.5 However, the role of SERCA3 in platelet function is poorly understood and its role in thrombus formation has not previously been studied.
Elaïb et al have now evaluated SERCA3−/− mice to determine the role of SERCA3 in platelet function. The authors demonstrated that although these mice breed normally and were phenotypically indistinguishable from littermates, they had abnormal platelet function. Their bleeding times were prolonged following tail clip and rates of rebleeding were markedly increased. They also displayed impaired thrombus formation in a ferric chloride-induced thrombosis model. SERCA3−/− platelets demonstrated decreased adhesion to collagen in flow chamber assays and impaired aggregation in response to low concentrations of either collagen or thrombin. Platelet dense granule release, as evidenced by decreased ATP release, and α-granule secretion were also impaired.
In evaluating the defect in SERCA3−/− platelet function, the authors noted that the addition of ADP to SERCA3−/− platelets reversed the defects in aggregation, adhesion, and secretion. Furthermore, the mutant platelets showed absolutely no defect in their ability to aggregate in response to ADP. Studies using a SERCA3-specific inhibitor (2,5-di-[tert-butyl]-1,4-benzohydroquinone; see figure) reproduced the effects of SERCA deletion on dense granule secretion. An inhibitor of SERCA2b (thapsigargin; see figure) did not elicit the same impairment of secretion. These observations suggested that the deficiency of ADP release was responsible for the defects in aggregation, adhesion, and α-granule secretion.
But why was ADP release impaired? The authors showed that dense granules were generally similar in morphology and serotonin content. Thus, SERCA3 deficiency does not appear to result in a dense granule storage defect. The authors also did not find colocalization of SERCA3 with dense granules. The fact that α-granule release was normal following supplementation with ADP ruled out a generalized secretory defect. Because SERCA3 serves a critical role in calcium metabolism, the authors evaluated the role of SERCA3-dependent calcium storage in platelet dense granule release.
They found that SERCA3−/− platelets stored lower levels of calcium in intracellular compartments than wild-type platelets. They also found that both calcium mobilization following thrombin exposure and calcium influx following exposure to extracellular calcium were impaired in SERCA3 null platelets. The defect in calcium mobilization was reversed upon incubation with exogenous ADP. Based on these observations, the authors concluded that SERCA3-dependent calcium storage is required for dense granule release at low agonist concentrations.
The ability to store calcium in intracellular compartments is critical for normal platelet function. Store-operated Ca2+ entry (SOCE) involves the coordinated activity of several proteins. Orai1, a channel protein capable of transporting calcium into cytosol, and STIM1, which senses intracellular calcium stores and stimulates Orai1 when stores are depleted, are known to mediate SOCE in platelets. Deletion of Orai1 in mice results in high rates of infant mortality, severely defective platelet SOCE, impaired platelet activation, and reduced thrombus formation.6 STIM1 deletions also profoundly affect infant mortality, platelet function, and thrombus formation.7 The studies of Elaïb et al demonstrate a much more subtle defect in SERCA3−/− mice. The mice breed normally and their platelet function defect is only observed at low concentrations of agonists. They also suggest a special role for SERCA3 in dense granule release.
The precise function of SERCA3 in dense granule release remains to be defined. Previous work showed that SERCA preferentially associates acidic calcium stores, thought to be lysosomal-related organelles such as dense granules and lysosomes.8 SERCA3 could reside on dense granule membranes and contribute to secretion. However, SERCA3 has not conclusively been localized to dense granules or lysosomes. An alternative possibility, not yet rigorously evaluated, is a physical association or proximity of dense granules to SERCA3-dependent calcium stores. Although calcium sensors mediate vesicle secretion in many cell types, the role of calcium sensors in controlling dense granule secretion is poorly understood. The intriguing observation that SERCA3 functions in dense granule release will provide new impetus to study the calcium dependency of platelet granule secretion.
Conflict-of-interest disclosure: The author declares no competing financial interests.