In this issue of Blood, Hinds et al identify several novel polymorphic genomic loci that are associated with an increased risk of developing JAK2 V617F–driven clonal hematopoiesis and myeloproliferative neoplasms (MPNs).1 Their findings affirm the notion that, although the acquisition of somatic mutations in hematopoietic stem cell (HSC) genomes is an infrequent and apparently stochastic process, the fate of mutant cells and their clonal progeny is profoundly influenced by heritable genetic polymorphisms present in the host’s genome.
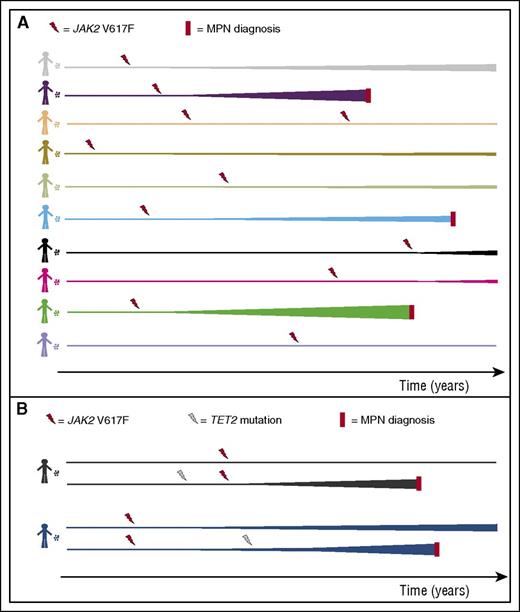
Impact of the inherited genome on the fate of JAK2 V617F mutant hematopoietic stem cells. (A) After the acquisition of the JAK2 V617F mutation, the fate of HSCs and their progeny is markedly influenced by heritable polymorphisms in the host genome. In many instances, expansion of JAK2 V617F–positive clones (depicted as expanding lines) leads to the development of detectable clonal hematopoiesis, and in some of these, the clones enlarge sufficiently to produce an MPN phenotype. It is also probable that, in many instances, clones remain very small and below the detection limits of conventional approaches. It should be noted that, although many genetic polymorphisms are likely to operate in a cell autonomous manner, others may influence the growth of mutant HSCs through non–cell autonomous effects. Also, the clonal size required to produce a clinical phenotype varies significantly between individuals, and this is also likely to be influenced by genetics. (B) Although most cases of JAK2 V617F–positive MPNs do not harbor any additional identifiable somatic driver mutations, some cases do have such mutations and they most commonly affect the TET2 gene. Hypothetically prior acquisition of a mutation in TET2 may be able to convert an unfavorable genome into a favorable one for JAK2 V617F to drive clonal expansion and MPN development (this is depicted in the fates of 2 distinct HSCs from the same individual). Alternatively, acquisition of TET2 mutations after JAK2 can accelerate clonal growth.
Impact of the inherited genome on the fate of JAK2 V617F mutant hematopoietic stem cells. (A) After the acquisition of the JAK2 V617F mutation, the fate of HSCs and their progeny is markedly influenced by heritable polymorphisms in the host genome. In many instances, expansion of JAK2 V617F–positive clones (depicted as expanding lines) leads to the development of detectable clonal hematopoiesis, and in some of these, the clones enlarge sufficiently to produce an MPN phenotype. It is also probable that, in many instances, clones remain very small and below the detection limits of conventional approaches. It should be noted that, although many genetic polymorphisms are likely to operate in a cell autonomous manner, others may influence the growth of mutant HSCs through non–cell autonomous effects. Also, the clonal size required to produce a clinical phenotype varies significantly between individuals, and this is also likely to be influenced by genetics. (B) Although most cases of JAK2 V617F–positive MPNs do not harbor any additional identifiable somatic driver mutations, some cases do have such mutations and they most commonly affect the TET2 gene. Hypothetically prior acquisition of a mutation in TET2 may be able to convert an unfavorable genome into a favorable one for JAK2 V617F to drive clonal expansion and MPN development (this is depicted in the fates of 2 distinct HSCs from the same individual). Alternatively, acquisition of TET2 mutations after JAK2 can accelerate clonal growth.
The JAK2 V617F mutation is present in almost all patients with polycythemia vera (PV) and more than half of those with essential thrombocytosis (ET) and primary myelofibrosis (PMF).2 More recently, the mutation was also identified as one of the most common drivers of age-related clonal hematopoiesis (ARCH),3 detected in >1% of hematologically normal people ≥60 years when sensitive methodology was used.4 By contrast, JAK2 V617F–driven MPNs are much rarer, with an estimated prevalence of 6 to 8 per 10 000.
As ARCH is the likely precursor of myeloid neoplasms, what are the determinants of progression of JAK2 V617F–driven ARCH to MPN? For malignancies such as acute myeloid leukemia (AML), progression from ARCH usually relies on the acquisition of additional mutations, and although this may also be true for some JAK2 V617F–positive MPNs, including many cases of PMF, JAK2 V617F is the sole identifiable driver mutation in the majority.2 In the context of this “clean” somatic genome, the progression from ARCH to MPN can be viewed as a race in which the host cell/genome represents a vehicle, which the JAK2 V617F mutation commandeers toward MPN. Continuing the metaphor, different vehicles (ie, different genomes) will be more or less able to get to the destination: MPN (see figure panel A). An important confounder here is the fact that the clinical presentation of MPNs occurs at different disease burdens in different people and is also influenced by the disease subtype (PV vs ET vs PMF), both of which affect the “distance” to the destination. Also the natural reduction in hemoglobin concentration in old age may further complicate this, as can the systemic and microenvironmental changes associated with aging.5
Previous studies identified genetic polymorphisms that are associated with an increased risk of JAK2 V617F–positive MPNs including common variants close to JAK2 itself, TERT and MYB.6 However, the study by Hinds et al significantly expands their number and identifies genes operating in diverse cellular processes including JAK/STAT signaling (SH2B3), DNA cytosine methylation (TET2), transcriptional regulation (GFI1B, PINT), and cell cycle control (CHEK2, ATM). Although these genes give biological insights into the major pathways involved in clonal expansion/progression, their diversity suggests that additional loci exist that influence risk of progression, albeit with effects that are not necessarily potent enough to be detected by studies performed thus far. Notably, the polymorphisms at JAK2 and TERT are also associated with an increased risk of JAK2-negative MPNs, indicating that other somatic mutations also can interact with the germline genome to drive clonal expansion. In this light, one might expect similar or equivalent polymorphisms to influence clonal growth in ARCH driven by mutations in genes such as DNMT3A, ASXL1, and TET2, although the fact that these mutations are not associated with a specific disease phenotype would have impeded detection of such an association to date.
Finally, the identification by Hinds et al of the same variants in association with both ARCH and MPN may be hinting at a potentially striking message. The unexpectedly high prevalence of ARCH4 had framed this phenomenon as representing the bulk of an iceberg of individuals with JAK2 V617F clones, with MPN cases being the tip. If this were true, one should expect the MPN cases to be those associating with genetic polymorphisms facilitating clonal expansion, but not ARCH. The fact that the identified variants are associated with an increased risk of both suggests that ARCH and MPN together may in fact represent the tip of the iceberg, with the bulk represented by much larger numbers of individuals with tiny JAK2 V617F clones that are undetectable by current approaches. If this is indeed true, it would support the premise that chance (acquisition of JAK2 V617F) is not necessarily the dominant factor in the development of MPN and that fate (having a favorable inherited genome) is potentially equally important. The acquisition of oncogenic mutations during a person’s lifetime was shown to be inevitable in sun-exposed skin,7 albeit in cells with large mutation burdens. If this inevitability were also true for the acquisition of JAK2 V617F by one or more HSCs, it would offer explanations for 2 intriguing observations: the familial occurrence of MPNs driven by somatically acquired mutations in JAK2, MPL, and CALR and the lack of an advantageous impact of JAK2 V617F on murine HSCs.8 In the latter instance, the mouse model can be seen as harboring an unfavorable genome for the full effects of JAK2 V617F, equivalent to humans who do not go on to develop ARCH after acquiring the mutation. Plausibly, the acquisition of prior mutations such as those affecting TET2 may be able to change an unfavorable genome to a favorable one (see figure panel B). In fact, a requirement to acquire a TET2 mutation before JAK2 V617F for clonal expansion to ensue would put a new light on the basis of phenotypic differences between “JAK2 first” and “TET2 first” double mutant MPNs.9
Therefore, what is the most important factor for the development of JAK2 V617F MPNs: the stochastic acquisition of the JAK2 V617F mutations in a person’s lifetime or the inheritance of a permissive genome? Based on what we know so far, the probable answer is that the two are similarly important, something that at first seems like a coincidence. However, on reflection, it may signify that this balance has “been appropriately constituted by an internal spontaneity” as “whatsoever things were not thus constituted, perished.”10
Conflict-of-interest disclosure: The author declares no competing financial interests.