To the editor:
Recently, Teira et al published a study for the Center for International Bone Marrow Transplant Research (CIBMTR) showing no benefit of cytomegalovirus (CMV) reactivation for relapse risk in acute myeloid leukemia (AML) patients after transplant.1 Unfortunately, in this register study, a uniform definition for CMV reactivation was not given, nor were the methods for evaluation of CMV reactivation described; so each participating center defined CMV reactivation differently with a heterogeneously defined cutoff level using different methods. Furthermore, it was not clear whether and how CMV reactivation was consequently treated with antiviral agents in each center as has been done in all other studies about CMV reactivation. This weakness in the study design seriously questions the reliability of the results of this study.1 In contrast, we and others have published that CMV reactivation correlates with substantially improved reduction of relapse incidence in patients with AML after transplant.2-10 The antileukemic effect of CMV reactivation is pronounced in AML and chronic myeloid leukemia (CML), but is detectable to a lesser extent in myelodysplastic syndrome (MDS), acute lymphoblastic leukemia (ALL), non-Hodgkin lymphoma, and pediatric acute leukemia.3-10 We defined this remarkable phenomenon as a virus-versus-leukemia effect, which is rare in hematology and contrary to the effects of oncovirus causing cancer or hematological malignancies such as hepatitis B and C virus, Epstein-Barr virus, human T-lymphotropic virus, Kaposi sarcoma–associated herpes virus, and human papilloma virus.11
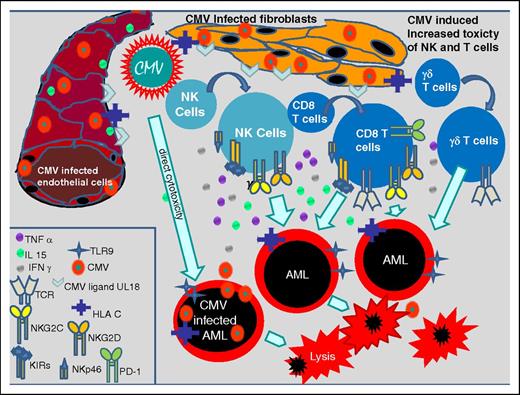
CMV infects a variety of cells such as endothelial cells, fibroblasts, retinal epithelial cells, bronchial and alveolar cells, neurons, hepatocytes, monocytes, dendritic cells, CD34+ progenitor cells, and AML cells and thereby commonly evokes innate and adaptive immune system responses.12 Innate immune responses to a CMV infection induce the proliferation of CMV-specific natural killer (NK) and T cells, which express activating and inhibitory killer cell immunoglobulin-like receptors (KIRs) and NK receptors as NKp46, NKG2C, NKG2D on their cell surface.13-16 Some of these NK cells are CD56dimCD57+NKG2C+, probably representing memory NK cells and may be therefore classified as part of the adaptive immune system.17 It has been shown that a CMV infection causes the exaggerated proliferation of γ/δ T cells and CD8+ T cells coexpressing CD56, CD57, and NKG2C too. NKG2C+ NK cells, NKG2C+ T cells, KIR-expressing NK cells, and γ/δ T cells persist over a longer time period after transplant and activate further cytotoxic T cells and NK cells, which probably counteract AML blasts by cross-reactivity and intensify the graft-versus-leukemia effect after transplant.13-21 Nevertheless, CMV also has direct cytotoxic effects in infected blasts, inducing apoptosis as shown recently.18 Figure 1 shows different CMV-induced antileukemic effects.
CMV-induced immune modulating effects. CMV-infected fibroblasts and endothelial cells inducing the expression of TCR, TLR9, CMV ligand U18, HLA-C, NKG2C, NKG2D, KIRs, NKp46, and PD-1 on NK cells and CD8 T cells, and proliferation of γδ+ T cells which increase their toxic effects on AML blasts. Further CMV mediates direct cytotoxicity on AML blasts. IFN, interferon; IL, interleukin; PD-1, programmed death 1; TCR, T-cell receptor; TLR, Toll-like receptor; TNF, tumor necrosis factor.
CMV-induced immune modulating effects. CMV-infected fibroblasts and endothelial cells inducing the expression of TCR, TLR9, CMV ligand U18, HLA-C, NKG2C, NKG2D, KIRs, NKp46, and PD-1 on NK cells and CD8 T cells, and proliferation of γδ+ T cells which increase their toxic effects on AML blasts. Further CMV mediates direct cytotoxicity on AML blasts. IFN, interferon; IL, interleukin; PD-1, programmed death 1; TCR, T-cell receptor; TLR, Toll-like receptor; TNF, tumor necrosis factor.
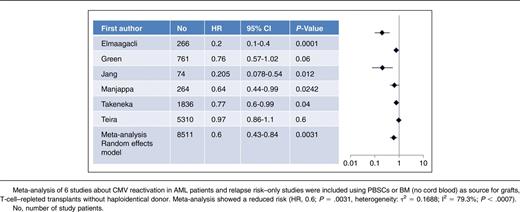
Here, we performed a meta-analysis of 6 studies with 8511 AML patients after transplant with peripheral blood stem cells (PBSCs) or bone marrow (BM) as grafts included (no cord blood as grafts) as listed in Table 1. Further inclusion criteria were mainly T-cell–repleted transplantation, a minimum of 60 study patients, HLA-compatible sibling or unrelated donors (up to 2 mismatched HLA loci allowed, no haploidentical transplants), no use of alemtuzumab for in vivo T-cell depletion.2-4,9,10,19 Meta-analysis was performed using EZR software22 from the European Society for Blood and Marrow Transplantation (EBMT; heterogeneity of τ2 = 0.1306; I2 = 85.8%, P < .0001, so that the result was given as a random-effects model and not a fixed-effect model). In 5 of 6 studies with mostly patients who received a non-T-cell–depleted transplant with a mainly myeloablative conditioning regimen, documented CMV reactivation was associated with a reduced risk of relapse.2-4,9,10 Only Teira et al for the CIBMTR found no benefit of CMV reactivation for relapse risk in AML patients as mentioned previously.1 However, the meta-analysis confirmed (random-effects model: hazard ratio [HR], 0.6; 95% confidence interval [CI]: 0.43-0.84; P = .0031) that CMV reactivation after transplant results in a substantial reduction of risk for relapse as shown in Table 1. According to the criteria to quote and grades of evidence this corresponds to a quality of evidence of level II.23 Moreover, results of 3 studies focusing on patients with AML in advanced stages2,4,8 confirmed a benefit of CMV reactivation in regard to leukemic relapse risk for these patients too. Patients with advanced stages of AML have an increased relapse risk of about 50% when transplanted from HLA-identical sibling or unrelated donors compared with only 15% to 30% of patients transplanted with AML in first complete remission.24,25
However, it is unclear whether CMV reactivation has an effect on nonrelapse mortality (NRM), overall survival (OS), and diseases other than AML. Teira et al, Green et al, and Takenaka et al found that CMV reactivation was associated with an increased NRM rate.1,3,4 Moreover, Takenaka et al and Teira et al reported that the OS rate was decreased.1,4 In contrast, Jang et al as well as our group found that, in single-center studies, CMV reactivation was associated with an improved OS rate.9 Green et al observed a reduced risk of relapse by day 100 among patients with AML after transplant, but not in patients with ALL, lymphoma, CML, and MDS, whereas at 1 year after transplant CMV reactivation was associated with reduced risk of relapse in all patients but without achieving statistical significance.3 The Japanese register study of Takenaka et al reported a reduced risk of relapse after CMV reactivation only in AML, but not in ALL, lymphoma, or MDS.4 This correlates best to our unpublished observations (A.H.E.). Recently, Ito et al reported a beneficial effect of CMV reactivation in 110 CML patients with regard to relapse.5 Manjappa et al found a beneficial effect of CMV reactivation only in AML patients after allogeneic hematopoietic stem cell transplantation with a myeloablative conditioning regimen,10 whereas Cichocki et al contrast that by reporting a protective effect of CMV viremia in a cohort of patients receiving reduced conditioning only.17 The study patients had received (in majority) cord blood transplants, which consisted of different immune cell subpopulations and may have had differences in the hematopoietic progenitor cell source, which might have influenced the CMV-induced immune reaction.
For the AML patient, CMV reactivation seems to be associated with a clear benefit regarding relapse risk as shown here in a meta-analysis including studies with mainly non-T-cell–depleted grafts. The effect is pronounced in patients with advanced stages of AML. Therefore, the observed slightly increased NRM rate of CMV reactivation might be more than counterbalanced by a meaningful reduction in relapse rate, which may result in a better OS for this subgroup of patients. The positive effect of CMV reactivation might be compromised by use of alemtuzumab or antithymocyte globulin as graft-versus-host disease prophylaxis, or transplants from a HLA-mismatched donor or a haploidentical donor. These patients often show delayed immune reconstitution and are often not able to induce proliferation of CMV-specific cells, which mediates the CMV-induced effects elaborating NK and T cells for a longer time period after transplant, therefore abolishing the immune-stimulating effects of CMV reactivation and thereby compromising the graft-versus-leukemia effect. CMV vaccination might induce similar immune-stimulating effects as CMV reactivation, but probably without inducing an increased NRM rate. This could result in improved OS, which should be investigated prospectively. However, licensed CMV vaccines are not yet available.
Authorship
Contribution: A.H.E. performed the meta-analysis and created the figure and table; and M.K. and A.H.E. wrote the text.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Ahmet H. Elmaagacli, Department of Hematology/Oncology and Stem Cell Transplantation, Helios Klinik Schwerin, Wismarsche Str 393-397, 19055 Schwerin, Germany; e-mail: ahmet.elmaagacli@uni-due.de.
References
Author notes
A.H.E. and M.K. contributed equally as authors.