In this issue of Blood, Morillo-Gutierrez et al report findings from the largest multicenter retrospective study on the use of treosulfan as the primary conditioning agent for hematopoietic stem cell transplantation (HSCT) in children with chronic granulomatous disease (CGD).1
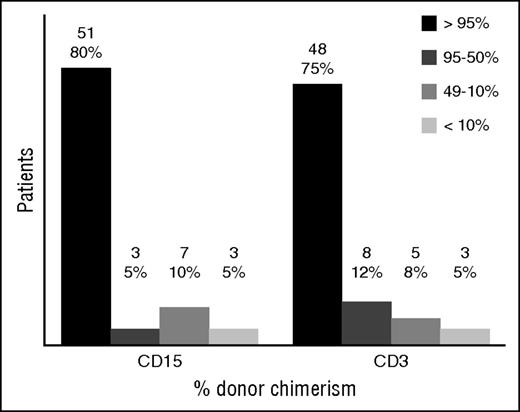
Split cell chimerism for CD15+ and CD3+ cells at last follow up. Results given in absolute number and percentage over the 64 patients with available split chimerism. Those who had second procedures were included with their last result before the event. Those who died had the last result available before their death. See Figure 3 in the article by Morillo-Gutierrez et al that begins on page 440.
Split cell chimerism for CD15+ and CD3+ cells at last follow up. Results given in absolute number and percentage over the 64 patients with available split chimerism. Those who had second procedures were included with their last result before the event. Those who died had the last result available before their death. See Figure 3 in the article by Morillo-Gutierrez et al that begins on page 440.
Their main discovery in the 70 patients studied is that treosulfan, a low toxicity alkylating agent, can be used effectively as part of conditioning for HSCT in high-risk children with CGD. This represents a major breakthrough for the treatment of this condition, which is complicated by a lifetime of serious bacterial and fungal infections that also put these patients at high risk for transplantation. In the few reported cases of toxicity in these authors’ study, it was minimal, and primarily involved the skin and resolved spontaneously. In particular, no veno-occlusive disease occurred, even in patients receiving a second transplant.
It has been recognized for some time that patients with genetic defects in the immune system do not require the same conditioning that patients with malignancies do, prior to HSCT. Many, if not most of these immunodeficient patients are already seriously and chronically infected by the time transplantation is considered, so their chances of surviving standard conditioning regimens are low. A variety of reduced-intensity conditioning regimens have, therefore, been tried for primary immunodeficiency patients undergoing HSCT, with less overall toxicity but with varying success in preventing autologous recovery or mixed chimerism.2 However, it is often not appreciated that the type of conditioning needed (if any) depends upon the genetic defect being treated. If the defect is primarily in T-lineage cells, the conditioning required is much less, as shown by the many patients with severe combined immunodeficiency (SCID) who had successful T-cell reconstitution with no3 or low-dose conditioning because their T-cell defects prevented graft rejection. Because SCID patients’ myeloid cells are usually normal in number and function, there is no need to achieve myeloid chimerism. However, in CGD, the defect is in the myeloid lineage, so the assumption has been that full myeloablative conditioning prior to HSCT is needed to cure this condition. Importantly, regarding the latter requirement, a major finding in the Morillo-Gutierrez et al study was that the level of chimerism achieved in both myeloid and T-cell lineages in these treosulfan-conditioned HSCT-treated CGD patients was excellent (see figure).
The authors rightfully caution, however, that long-term follow up is required to compare with other regimens and to ascertain late effects of the agent, particularly on gonadal function. The largest prospective study of reduced-intensity conditioning for CGD, published by Güngör et al4 in 2014, included 56 adults and children from 16 centers, and used sub-myeloablative doses of busulfan, an alkylating agent known to have greater toxicity than treosulfan. The results of the latter study compare favorably with the results of the Morillo-Gutierrez et al study. The 21-month median overall survival (OS) and event-free survival (EFS) rates in that study were 93% and 89%, respectively, similar to the OS and EFS rates of 91.4% and 81.4% found by Morillo-Gutierrez et al, at a median follow-up of 34 months. The cumulative incidence of acute grade 3-4 graft-versus-host disease (GVHD) was 4% in the Güngör at al4 study compared with 12% in the Morillo-Gutierrez et al study. Nine patients (13%) developed chronic GVHD in the Morillo-Gutierrez et al study (although 6 of them later became asymptomatic) and 4 patients (7%) in the Güngör et al4 study. Graft failure occurred in 5% of patients in the Güngör et al4 study and in 12% of patients in the Morillo-Gutierrez et al study. Stable (≥90%) donor myeloid chimerism was documented in 93% of the surviving patients in the Güngör et al4 study.
The problems with analyzing data from the above and all other transplant outcome studies, retrospective or prospective, are the concomitant use of other conditioning agents and the use of various types of donors. In the Morillo-Gutierrez et al study, it could be shown that that there were no differences between matched and mismatched donor grafts in either OS or EFS, and the addition of thiotepa had no effect. The increasing use of serotherapy, however, is another variable that confounds the outcomes of HSCT studies. Serotherapy agents, such as antithymocyte globulin (ATG) and alemtuzumab, are used primarily to enhance engraftment and suppress GVHD. However, the long-term effects of use of these agents need to be carefully studied, particularly for alemtuzumab. In a recent study of different doses of alemtuzumab, patients receiving the higher doses had lower lymphocyte counts at days 30 and 100 than did those receiving lower doses.5 It is well-recognized that the use of this agent is associated with a high incidence of viral reactivation posttransplantation.6 In addition, Dvorak et al and others have observed long-term delays in T-cell recovery and in the production of recent thymic emigrants in patients receiving this agent.7 In the Morillo-Gutierrez et al study, 57 patients received either ATG or alemtuzumab compared with 13 who did not receive any serotherapy and there was no significant effect of the use of these agents on the outcome measures, but no separate analysis was presented on the effects of use of alemtuzumab.
Finally, one of the well-recognized late toxicities of standard conditioning regimens is gonadal dysfunction. In a recent comparison study of busulfan-containing regimens vs reduced-intensity regimens containing other agents, there was a much higher incidence of gonadal dysfunction in those receiving busulfan-containing regimens.4 It will take several years before it is known whether treosulfan causes this problem.
Conflict-of-interest disclosure: The author declares no competing financial interests.