Key Points
Malignant cells from patients with AML expose danger signals on the plasma membrane regardless of chemotherapy.
Such danger signals correlate with markers of a clinically relevant tumor-specific immune response and with improved disease outcome.
Abstract
Cancer cell death can be perceived as immunogenic by the host only when malignant cells emit immunostimulatory signals (so-called “damage-associated molecular patterns,” DAMPs), as they die in the context of failing adaptive responses to stress. Accumulating preclinical and clinical evidence indicates that the capacity of immunogenic cell death to (re-)activate an anticancer immune response is key to the success of various chemo- and radiotherapeutic regimens. Malignant blasts from patients with acute myeloid leukemia (AML) exposed multiple DAMPs, including calreticulin (CRT), heat-shock protein 70 (HSP70), and HSP90 on their plasma membrane irrespective of treatment. In these patients, high levels of surface-exposed CRT correlated with an increased proportion of natural killer cells and effector memory CD4+ and CD8+ T cells in the periphery. Moreover, CRT exposure on the plasma membrane of malignant blasts positively correlated with the frequency of circulating T cells specific for leukemia-associated antigens, indicating that ecto-CRT favors the initiation of anticancer immunity in patients with AML. Finally, although the levels of ecto-HSP70, ecto-HSP90, and ecto-CRT were all associated with improved relapse-free survival, only CRT exposure significantly correlated with superior overall survival. Thus, CRT exposure represents a novel powerful prognostic biomarker for patients with AML, reflecting the activation of a clinically relevant AML-specific immune response.
Introduction
For nearly a century, cancer has been viewed as an immunologically silent entity that should be treated with high-dose chemotherapy or radiation therapy, pretty much as a bacterial infection to be eradicated with potent antibiotics.1,2 The limitations of such a view became clear throughout the past decade, as several laboratories worldwide demonstrated that tumors arise, become clinically manifest, and respond to treatment in the context of a bidirectional crosstalk with the host immune system.1-4 One of the mechanisms whereby neoplastic cells succumbing to specific treatments can activate the immune system is commonly referred to as “immunogenic cell death” (ICD).5-7 Thus, malignant cells exposed to some chemotherapeutic agents like anthracyclines, oxaliplatin and bortezomib, as well as to fractionated radiation therapy or high hydrostatic pressures, succumb as they expose (on their surface) or release (in the extracellular milieu) a set of molecules that alert the immune system of incipient danger.8-11 Importantly, the emission of such danger signals, which altogether are known as damage-associated molecular patterns (DAMPs), mechanistically relies on the activation of adaptive stress responses in dying cells, and hence, can be pharmacologically modulated.12
ICD-relevant DAMPs encompass but are not limited to the following13,14 : (1) the exposure of endoplasmic reticulum (ER) chaperones like calreticulin (CALR, best known as CRT), heat shock protein family A (Hsp70) member 1A (HSPA1A, best known as HSP70), and heat shock protein 90 α family class A member 1 (HSP90AA1, best known as HSP90) on the plasma membrane15,16 ; (2) the secretion of adenosine triphosphate (ATP), annexin A1 (ANXA1), and high mobility group box 1 (HMGB1) in the extracellular microenvironment17,18 ; and (3) the activation of autocrine/paracrine type I interferon (IFN) signaling in malignant cells, eventually resulting in the secretion of C-X-C motif chemokine ligand 10 (CXCL10).19
DAMPs mediate robust immunostimulatory effects upon interaction with pattern recognition receptors (PRRs) expressed on myeloid or lymphoid cells,20 including LDL receptor related protein 1 (LRP1, best known as CD91), which binds CRT, HSP70, and HSP90; purinergic receptor P2X7 (P2RX7) and purginergic receptor P2Y2 (P2RY2), which bind ATP; formyl peptide receptor 1, which binds ANXA1; Toll-like receptor 4, which binds HMGB1; and C-X-C motif chemokine receptor 3 (CXCR3), the T-cell receptor for CXCL10.13 In particular, surface-exposed (ecto)-CRT, ecto-HSP70, and ecto-HSP90 foster the uptake of dead cancer cells or their corpses; ATP stimulates the recruitment of myeloid cells to the tumor microenvironment (via P2RY2) and their activation (via P2RX7), and ANXA1 guides the homing of myeloid cells toward dying cancer cells. HMGB1 has multipronged proinflammatory functions, and CXCL10 favors T-cell infiltration.1
Corroborating the clinical relevance of ICD and its proper perception, several DAMPs and DAMP-associated parameters (eg, DAMP and PRR expression levels, genetic polymorphisms in DAMP- or PRR-coding genes, etc) have been attributed prognostic value in several cohorts of patients with cancer.21 In particular, CRT levels or exposure has been shown to convey robust prognostic information in subjects with multiple solid tumors, including neuroblastoma, non–small cell lung carcinoma, ovarian cancer, and colorectal carcinoma.22-25 However, little is known on the prognostic value of CRT in patients with hematological malignancies, irrespective of the fact that these patients often receive ICD inducers, including anthracyclines and bortezomib. In a preliminary study, we reported that malignant blasts from patients with acute myeloid leukemia (AML) may expose CRT on the plasma membrane regardless of chemotherapy,26 but did not investigate the prognostic relevance of these findings. Driven by these premises, we decided to investigate the immunological and prognostic correlates of DAMP emission in an independent cohort of patients with AML, finding that CRT exposure (and less so HSP70 and HSP90 exposure) on malignant blasts correlates with the activation of tumor-targeting immune responses and improved clinical outcome.
Materials and methods
Patients and samples
Fifty patients with a confirmed diagnosis of AML who were treated at the Institute of Hematology and Blood Transfusion of Prague, Czech Republic between January 2013 and July 2015 were enrolled in this study. Informed consent was obtained according to the Declaration of Helsinki, and the study was approved by the local ethics committee. The main clinical and biological characteristics of the patients are summarized in Table 1. Peripheral blood samples were obtained before chemotherapy, 12 hours after the initiation of chemotherapy, and once blood cell counts were restored after chemotherapy (50-70 days after chemotherapy). Serum was separated and stored at −80°C, and peripheral blood mononuclear cells (PBMCs) were isolated by Ficoll density centrifugation (GE Healthcare). An EasySep kit (StemCell Technologies) was employed to separate normal PBMC from CD33+ malignant blasts.
Cell culture
Patient-derived PBMCs were cultured in RPMI 1640 medium (Life Technologies) supplemented with 10% heat-inactivated pooled human AB serum, 100 U/mL penicillin, 2 mM L-glutamine, nonessential amino acids, and sodium pyruvate (Life Technologies). Kasumi-1 and MOLM-13 cells were maintained in RPMI 1640 medium supplemented with 20% (Kasumi-1) and 10% (MOLM-13) fetal bovine serum, 100 U/mL penicillin, and 2 mM L-glutamine.
Flow cytometry
For the analysis of CRT, HSP70, or HSP90 exposure, PBMCs were labeled with primary anti-CD45 (PerCP; BD Bioscience), anti-CD33 (PE; BD Bioscience), and either anti-CRT (Enzo Life Sciences), anti-HSP70 (R&D Systems), or anti-HSP90 (R&D Systems) antibodies for 20 minutes at 4°C, followed by 1 wash in phosphate-buffered saline and incubation with an antigen-presenting cell (APC)-conjugated secondary antibody (Jackson Immunoresearch). Kasumi-1 and MOLM-13 cells were stained according to the same protocol in the absence of primary anti-CD45 and anti-CD33 antibodies. In both cases, cell suspensions were eventually costained with fluorescein isothiocyanate–conjugated annexin V (Exbio Antibodies) plus 4',6-diamidino-2-phenylindole (DAPI). For the assessment of dendritic cell (DC) activation, monocyte-derived DCs pulsed with Kasumi-1 cells succumbing to idarubicin or daunorubicin were stained with CD86-PE-Cy5 (Exbio Antibodies) and HLA-DR-PE-Cy7 (BD Biosciences). Flow cytometry data were acquired on an LSRFortessa Analyzer (BD) and analyzed with the FlowJo software package (Tree Star, Inc). Ecto-CRT, ecto-HSP70, and ecto-HSP90 were monitored on DAPI− (living) cells, as per gold-standard procedures.27,28
Phagocytosis
DCs were generated by culturing purified CD14+ cells isolated from buffy coats in the presence of 500 IU/mL granulocyte-macrophage colony-stimulating factor and 248 IU/mL interleukin-4 (IL-4) (both Gentaur). On day 5, immature DCs stained with Vybrant DiD cell labeling solution (Life Technologies) were fed Kasumi-1 cells previously exposed to 20 µM idarubicin or 20 µM daunorubicin for 24 hours (at a 5:1 DC/tumor cell ratio) and stained with VybrantV DiO cell labeling solution (Life Technologies). After 24 hours at either 37°C or 4°C, phagocytosis was analyzed by flow cytometry as the percentage of DiO+DiD+ events.
Enzyme-linked immunosorbent assay
Circulating levels of CRT, HSP70, HSP90, and HMGB1 were assessed on frozen sera by enzyme-linked immunosorbent assay with commercial kits (Enzo Life Sciences or IBL), according to the manufacturer’s instructions.
Detection of antigen-specific T cells
The frequency of T cells specific for baculoviral IAP repeat containing 5 (BIRC5; best known as survivin), cyclin B1 (CCNB1), preferentially expressed antigen in melanoma (PRAME), and Wilms tumor 1 (WT1) was assessed by flow cytometry according to standard methods. In brief, PBMCs were cultured for 10 days together with mixtures of overlapping peptides (PepMix; JPT Peptide Technologies) spanning the whole sequence of BIRC5, CCNB1, PRAME, or WT1 at a final concentration of 1 µg/mL. On days 4 and 7, 20 UI/mL IL-2 (Gentaur) was added. On day 9, PBMCs were restimulated with peptide mixtures, and 5 µg/mL brefeldin A (BioLegend) was added after 4 hours of incubation. Alternatively, PBMCs were exposed to immature DCs alone or immature DCs pulsed with (1) 25 µg/mL polyinosinic-polycytidylic acid, (2) Kasumi-1 cells succumbing to 20 µM idarubicin, or (3) Kasumi-1 cells succumbing to 20 µM daunorubicin, for a total time of 14 days (restimulated twice). Eventually, PBMCs were costained with CD3-PE-Cy5, CD4-PE-Cy7 (eBioscience), and CD8-PE-Dy590 (Exbio) conjugates plus the Aqua Blue Live/Dead cell viability dye (Life Technologies). Thereafter, cells were fixed with fixation/permeabilization buffer (BD Bioscience), permeabilized with permeabilization buffer (BD Bioscience), and incubated with a fluorescein isothiocyanate–conjugated antibody specific for IFN-γ plus an APC-conjugated antibody specific for IL-2 (both from BD Bioscience), according to the manufacturer’s instructions. Flow cytometry was performed on an LSRFortessa Analyzer (BD), and data were analyzed with the FlowJo software package (Tree Star, Inc). Upon exclusion of dead cells, IFN-γ expression was considered to be antigen specific if the frequency of IFN-γ+ T cells detected in response to peptide stimulation was at least twice the frequency of IFN-γ+ cells detected in control conditions.
Statistical analyses
Survival analyses were performed upon patient stratification into 2 groups based on median levels of ecto-CRT, ecto-HSP70, ecto-HSP90, circulating HMGB1, or CALR messenger RNA (mRNA) levels. As an alternative, patients were stratified based on BIRC5, BMI1 proto-oncogene, polycomb ring finger (BMI1), CCNB1, elastase, neutrophil expressed (ELANE), hyaluronan-mediated motility receptor (HMMR, also known as RHAMM), MOK protein kinase (MOK, also known as RAGE1), membrane palmitoylated protein 1 (MPP1), PRAME, proteinase 3 (PRTN3), or WT1 expression levels. Univariate and multivariate Cox proportional hazard analysis was performed to assess the association of clinicopathological or immunological parameters with relapse-free (RFS) or overall survival (OS). Fisher’s exact test, Student t test, Wilcoxon, and Mann-Whitney tests were used to assess statistical significance. P values are reported (and were considered not significant when >.05).
Additional Materials and Methods are available as supplemental information, available on the Blood Web site.
Results
AML blasts emit DAMPs regardless of chemotherapy
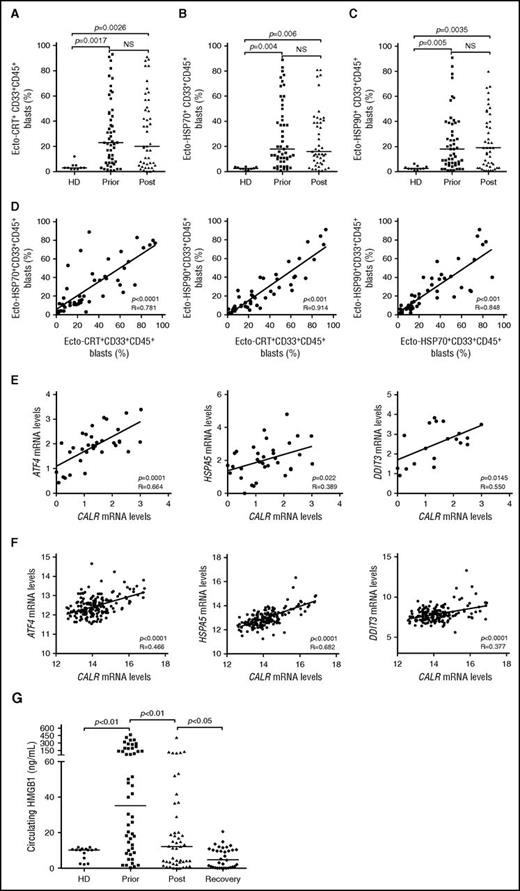
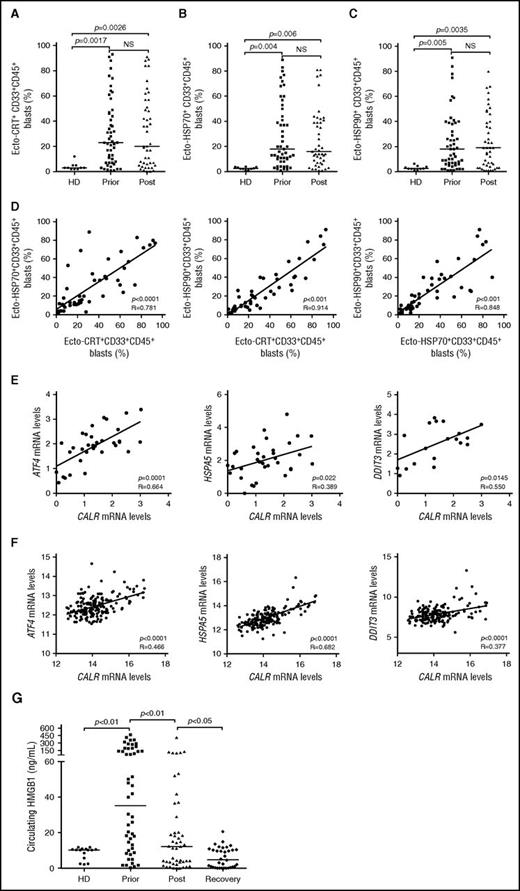
To expand our previous observations,26 we used flow cytometry to investigate the exposure of CRT, HSP70, and HSP90 on the plasma membrane of CD33+ malignant blasts from 50 patients with AML prior to and after induction anthracycline-based chemotherapy (Table 1; supplemental Figure 1). Forty-one patients with AML (82%) exhibited >5% circulating CD45+CD33+ blasts with surface-exposed CRT prior to the initiation of treatment, whereas PBMCs from 10 healthy donors contained (on average) <5% ecto-CRT+ cells (Figure 1A). Of note, the percentage of living (DAPI−) blasts staining positively for ecto-CRT was highly heterogeneous within the cohort, ranging from 5% to 95% of the CD45+CD33+ cell population (Figure 1A). Along similar lines, the blasts of patients with AML stained positively for ecto-HSP70 and ecto-HSP90 in a rather heterogeneous fashion, contrasting with living (DAPI−) PBMCs from healthy donors that never contained >5% ecto-HSP70+ or ecto-HSP90+ cells (Figure 1B-C). Indeed, CRT, HSP70, and HSP90 exposure on malignant blasts exhibited considerable mutual correlation (Figure 1D). The percentage of living (DAPI−) ecto-CRT+, ecto-HSP70+, and ecto-HSP90+ blasts was not influenced by disease subtype (supplemental Figure 2A-C). Of note, IV anthracycline-based chemotherapy failed to increase the percentage of living (DAPI−) blasts exposing CRT, HSP70, or HSP90 on their surface (Figure 1A-C), suggesting that in this specific setting DAMP emission may reflect a treatment-independent, cancer-cell intrinsic state of stress. No difference in the percentage of living (DAPI−) ecto-CRT+ blasts was observed in patients receiving idarubicin-based vs daunorubicin-based chemotherapy (supplemental Figure 2D). Accordingly, idarubicin and daunorubicin induced comparable degrees of CRT exposure in cultured human Kasumi-1 and MOLM-13 AML cells (supplemental Figure 2E), resulting in comparable phagocytosis of AML cells by cocultured myeloid cells (supplemental Figure 2F), similar expression of activation markers by the latter (supplemental Figure 2G), and comparable ability to drive the accumulation of IFN-γ+ CD4+ or CD8+ T cells (supplemental Figure 2H-I).
Chemotherapy-independent emission of danger signals by malignant AML blasts. (A-C) Percentage of ecto-CRT+ (A), ecto-HSP70+ (B), or ecto-HSP90+ (C) CD45+CD33+ cells from 10 healthy donors (HD) or 50 patients with AML before (Prior) or after (Post) the initiation of induction chemotherapy. Median values are reported. NS, nonsignificant. (D) Correlation between the percentage of ecto-CRT+, ecto-HSP70+, and ecto-HSP90+ CD45+CD33+ cells measured in 50 patients with AML before induction chemotherapy. R, Pearson correlation coefficient. (E) Correlation between CALR mRNA levels and ATF4, HSP5A, or DDIT3 mRNA levels in 34 patients with AML before induction chemotherapy. (F) Correlation between CALR mRNA levels and ATF4, HSP5A, or DDIT3 mRNA levels in 173 patients with AML from the TGCA public database. (G) Circulating HMGB1 levels from 10 HD or 50 patients with AML before the initiation of induction chemotherapy (Prior), 12 hours after induction chemotherapy (Post), or at the reestablishment of normal hematopoiesis (Recovery). Median values are indicated.
Chemotherapy-independent emission of danger signals by malignant AML blasts. (A-C) Percentage of ecto-CRT+ (A), ecto-HSP70+ (B), or ecto-HSP90+ (C) CD45+CD33+ cells from 10 healthy donors (HD) or 50 patients with AML before (Prior) or after (Post) the initiation of induction chemotherapy. Median values are reported. NS, nonsignificant. (D) Correlation between the percentage of ecto-CRT+, ecto-HSP70+, and ecto-HSP90+ CD45+CD33+ cells measured in 50 patients with AML before induction chemotherapy. R, Pearson correlation coefficient. (E) Correlation between CALR mRNA levels and ATF4, HSP5A, or DDIT3 mRNA levels in 34 patients with AML before induction chemotherapy. (F) Correlation between CALR mRNA levels and ATF4, HSP5A, or DDIT3 mRNA levels in 173 patients with AML from the TGCA public database. (G) Circulating HMGB1 levels from 10 HD or 50 patients with AML before the initiation of induction chemotherapy (Prior), 12 hours after induction chemotherapy (Post), or at the reestablishment of normal hematopoiesis (Recovery). Median values are indicated.
Because CRT expression relies on premortem ER stress responses,6 we checked whether the mRNA levels of 3 distinct genes intimately involved in this process,29 namely, activating transcription factor 4 (ATF4), heat shock protein family A (Hsp70) member 5 (HSPA5), and DNA damage inducible transcript 3 (DDIT3), would correlate with CRT exposure on malignant blasts. We observed a positive correlation between the percentage of living (DAPI−) ecto-CRT+ blasts and ATF4, HSPA5, and DDIT3 mRNA levels (Figure 1E). To corroborate our findings in an independent patient cohort, we retrieved normalized ATF4, HSPA5, and DDIT3 expression levels for 173 patients with AML from The Cancer Genome Atlas (TCGA) public database and analyzed their correlation with CALR mRNA abundance. Also, in this setting, ATF4, HSPA5, and DDIT3 mRNA levels exhibited a highly significant positive correlation with CALR expression (Figure 1F), corroborating the notion that AML blasts are subjected to high levels of ER stress irrespective of treatment, resulting in spontaneous CRT exposure in a majority of patients.
Next, we monitored the levels of circulating ICD-related DAMPs (including HMGB1, CRT, HSP70, and HSP90), in the sera of healthy volunteers and patients with AML prior to induction chemotherapy, 12 hours after induction chemotherapy, and at recovery. All these circulating DAMPs were more abundant in patients with AML before treatment than in healthy individuals (Figure 1G; supplemental Figure 3A-C). Although the amount of CRT and HSP70 did not change significantly throughout the study, patients with AML exhibited a reduction in circulating HMGB1 (and HSP90) levels as they recovered normal blood counts (Figure 1G; supplemental Figure 3C). This finding may simply reflect the reduction in circulating blasts spontaneously releasing HMGB1 (and HSP90) imposed by chemotherapy. Of note, we failed to identify a correlation between the percentage of living (DAPI−) ecto-CRT+, ecto-HSP70+, and ecto-HSP90+ blasts and the serum concentration of CRT, HSP70, and HSP90, respectively (supplemental Figure 3D). Moreover, disease subtype did not influence the circulating levels of HMGB1, CRT, HSP70, and HSP90 (supplemental Figure 4A-D).
CRT exposure is associated with markers of a TH1 cytotoxic CD8+ T-cell response upon treatment
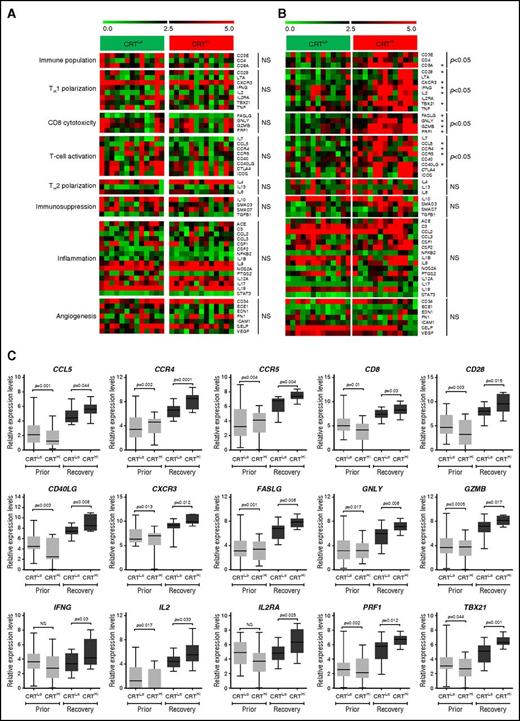
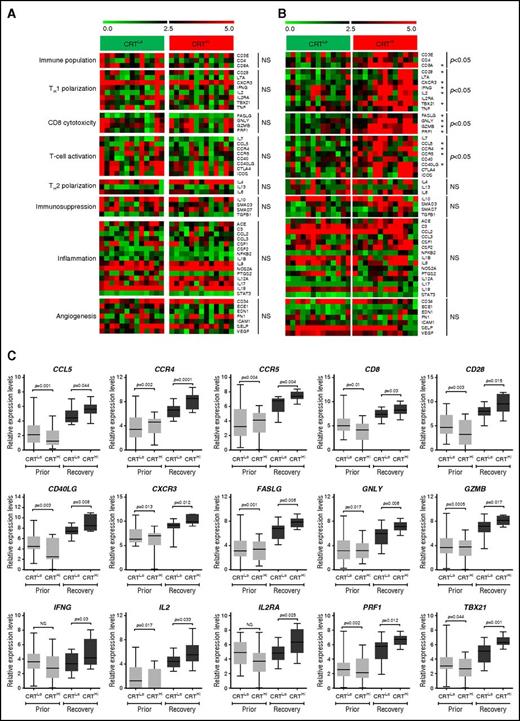
Because ecto-CRT is best known for its ability to promote the phagocytosis of dying cancer cells by APCs and hence initiate antitumor immunity, we set out to evaluate the expression of immune system–related genes in normal (CD33−) PBMCs from 26 patients with AML. Patients were stratified based on the median percentage of living (DAPI−) ecto-CRT+ blasts into a CRTHi (n = 13) and a CRTLo (n = 13) group. In baseline conditions (prior to induction chemotherapy), we were unable to identify statistically significant differences between the expression of immune cell population-, TH1 polarization-, TH2 polarization-, CD8+ T-cell cytotoxicity-, T-cell activation-, immunosuppression-, inflammation-, and angiogenesis-related gene clusters in CRTHi vs CRTLo patients (Figure 2A). Conversely, upon complete remission and the recovery of normal, nonmalignant hematopoiesis, the gene clusters related to immune cell populations, TH1 polarization, CD8+ T-cell cytotoxicity, and T-cell activation were considerably upregulated in CRTHi vs CRTLo patients with AML (Figure 2B). The expression of gene clusters related to TH2 polarization, immunosuppression, inflammation, and angiogenesis did not differ between CRTHi and CRTLo patients with AML even at recovery (Figure 2B). A correlation matrix was built to confirm clustering (supplemental Figure 5). Moreover, we validated the majority of these findings by analyzing the expression levels of CD8A, CD28, CXCR3, IFNG, T-box 21 (TBX21, best known as T-bet), Fas ligand (FASLG), granulysin (GNLY), granzyme B (GZMB), perforin 1 (PRF1), C-C motif chemokine ligand 5 (CCL5), C-C motif chemokine receptor 4 (CCR4), CCR5, and CD40 ligand (CD40LG) by quantitative reverse transcription polymerase chain reaction (qRT-PCR) (Figure 2C).
Transcriptional signatures of PBMCs in patients with AML exhibiting robust vs weak CRT exposure on blasts. (A-B) Expression levels of genes from the Human Immune Panel TaqMan low-density array in PBMCs from 13 CRTHi vs 13 CRTLo patients with AML prior to the initiation of induction chemotherapy (A), or at recovery of normal hematopoiesis (B). The Mann-Whitney test was employed to assess intergroup variations. (C) qRT-PCR–assisted quantification of CD8A, CD28, CXCR3, IFNG, IL2, IL2RA, TBX21, FASLG, GNLY, GZMB, PRF1, CCL5, CCR4, CCR4, CCR5, CD40LG expression levels in PBMCs from 13 CRTHi vs 13 CRTLo patients with AML prior to the initiation of induction chemotherapy, or at recovery of normal hematopoiesis. Box plots: lower quartile, median, upper quartile; whiskers, minimum value, maximum value.
Transcriptional signatures of PBMCs in patients with AML exhibiting robust vs weak CRT exposure on blasts. (A-B) Expression levels of genes from the Human Immune Panel TaqMan low-density array in PBMCs from 13 CRTHi vs 13 CRTLo patients with AML prior to the initiation of induction chemotherapy (A), or at recovery of normal hematopoiesis (B). The Mann-Whitney test was employed to assess intergroup variations. (C) qRT-PCR–assisted quantification of CD8A, CD28, CXCR3, IFNG, IL2, IL2RA, TBX21, FASLG, GNLY, GZMB, PRF1, CCL5, CCR4, CCR4, CCR5, CD40LG expression levels in PBMCs from 13 CRTHi vs 13 CRTLo patients with AML prior to the initiation of induction chemotherapy, or at recovery of normal hematopoiesis. Box plots: lower quartile, median, upper quartile; whiskers, minimum value, maximum value.
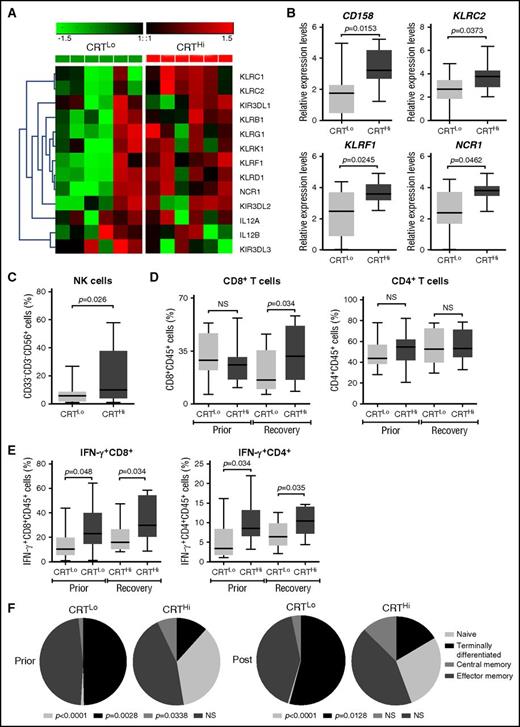
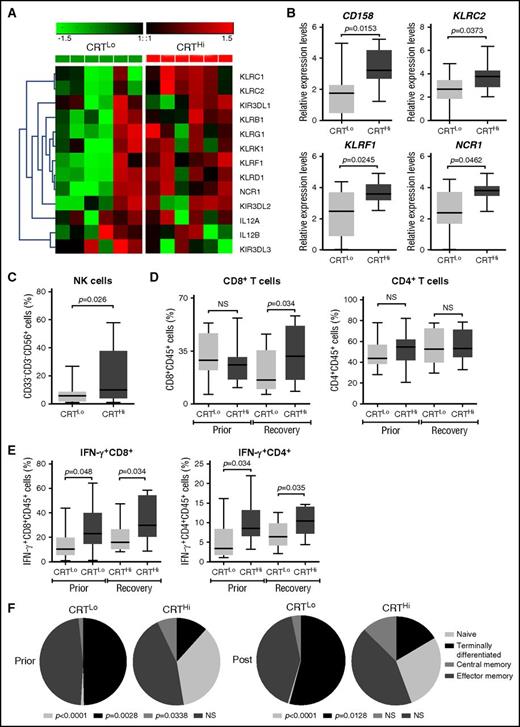
To extend these findings to other genes potentially involved in anticancer immunity, we employed the Nanostring technology to assess the relative abundance of 770 cancer and immune system mRNAs in the PBMCs of 6 CRTHi and 6 CRTLo patients with AML upon recovery of normal hematopoiesis. We identified a cluster of natural killer (NK) cell-related genes that was significantly overexpressed in CRTHi vs CRTLo patients (Figure 3A). We validated these results by qRT-PCR in a larger group of patients (20 CRTHi and 20 CRTLo individuals), confirming that several members of the CD158 gene family (namely, KIR2DL1, KIR2DL2, and KIR2DL3), killer cell lectin–like receptor F1 (KLRF1), killer cell lectin–like receptor C2 (KLRC2), and natural cytotoxicity triggering receptor 1 (NCR1, also known as NK-p46) were significantly overexpressed in CRTHi patients with AML as compared with their CRTLo counterparts (Figure 3B). In line with this notion, we detected an increased percentage of CD33−CD3−CD56+ NK cells in the blood of CRTHi vs CRTLo patients recovering normal hematopoiesis after chemotherapy (Figure 3C). Interestingly, the relative abundance of CD4+ and CD8+ T cells virtually did not differ in these 2 patient subgroups, except for a slightly higher proportion of CD3+CD8+ T lymphocytes in CRTHi patients with AML at recovery (Figure 3D). Conversely, CRTHi patients with AML had an increased percentage of CD4+ and CD8+ T cells responding with IFN-γ production to nonspecific stimulation (with phorbol 12-myristate 13-acetate plus ionomycin) as compared with their CRTLo counterparts, both in baseline conditions (before induction chemotherapy) and at recovery (Figure 3E). Moreover, whereas there was no difference in the proportion of circulating CD45RA−CD45RO+CCR7−CD62L− effector memory T cells between these patient subgroups, CRTLo patients exhibited a high frequency of CD45RA+CD45RO−CCR7−CD62L− terminally differentiated T cells, whereas CRTHi individuals manifested an increased abundance of CD45RA+CD45RO−CCR7+CD62L+ naive and CD45RA−CD45RO+CCR7+CD62L+ central memory T cells (Figure 3F).
Transcriptional and phenotypic signatures of PBMCs in patients with AML exhibiting robust vs weak CRT exposure on blasts. (A) Nanostring-assisted quantification of NK cell–related mRNAs in PBMCs from 6 CRTHi vs 6 CRTLo patients with AML at recovery of normal hematopoiesis. The Mann-Whitney test was employed to assess intergroup variations. (B) qRT-PCR–assisted quantification of KIR2DL1, KIR2DL2, and KIR2DL3 (cumulatively as CD158 family members), KLRF1, KLRC2, and NCR1 in PBMCs from 20 CRTHi vs 20 CRTLo patients with AML at recovery of normal hematopoiesis. Box plots: lower quartile, median, upper quartile; whiskers, minimum value, maximum value. (C-D) Percentage of circulating CD33−CD3−CD56+ NK cells (C), CD45+CD3+CD4+ T cells, and CD45+CD3+CD8+ T cells in 13 CRTHi vs 13 CRTLo patients with AML before induction chemotherapy (Prior, D) and at reestablishment of normal hematopoiesis (Recovery, C-D). Box plots: lower quartile, median, upper quartile; whiskers, minimum value, maximum value. (E) Percentage of IFN-γ–producing cells among CD45+CD3+CD4+ T cells and CD45+CD3+CD8+ T cells from 13 CRTHi vs 13 CRTLo patients with AML before induction chemotherapy (Prior) and at reestablishment of normal hematopoiesis (Recovery). Box plots: lower quartile, median, upper quartile; whiskers, minimum value, maximum value. (F) Distribution of circulating CD45+CD3+CD8+ T cells and CD45+CD3+CD4+ T cells in 20 CRTHi vs 20 CRTLo patients with AML before (Prior) and after (Post) induction chemotherapy. Mean percentage values are depicted as pie charts. Central memory: CD45RA−CD45RO+CCR7+CD62L+; effector memory: CD45RA−CD45RO+CCR7−CD62L−; naive: CD45RA+CD45RO−CCR7+CD62L+; terminally differentiated: CD45RA+CD45RO−CCR7−CD62L−.
Transcriptional and phenotypic signatures of PBMCs in patients with AML exhibiting robust vs weak CRT exposure on blasts. (A) Nanostring-assisted quantification of NK cell–related mRNAs in PBMCs from 6 CRTHi vs 6 CRTLo patients with AML at recovery of normal hematopoiesis. The Mann-Whitney test was employed to assess intergroup variations. (B) qRT-PCR–assisted quantification of KIR2DL1, KIR2DL2, and KIR2DL3 (cumulatively as CD158 family members), KLRF1, KLRC2, and NCR1 in PBMCs from 20 CRTHi vs 20 CRTLo patients with AML at recovery of normal hematopoiesis. Box plots: lower quartile, median, upper quartile; whiskers, minimum value, maximum value. (C-D) Percentage of circulating CD33−CD3−CD56+ NK cells (C), CD45+CD3+CD4+ T cells, and CD45+CD3+CD8+ T cells in 13 CRTHi vs 13 CRTLo patients with AML before induction chemotherapy (Prior, D) and at reestablishment of normal hematopoiesis (Recovery, C-D). Box plots: lower quartile, median, upper quartile; whiskers, minimum value, maximum value. (E) Percentage of IFN-γ–producing cells among CD45+CD3+CD4+ T cells and CD45+CD3+CD8+ T cells from 13 CRTHi vs 13 CRTLo patients with AML before induction chemotherapy (Prior) and at reestablishment of normal hematopoiesis (Recovery). Box plots: lower quartile, median, upper quartile; whiskers, minimum value, maximum value. (F) Distribution of circulating CD45+CD3+CD8+ T cells and CD45+CD3+CD4+ T cells in 20 CRTHi vs 20 CRTLo patients with AML before (Prior) and after (Post) induction chemotherapy. Mean percentage values are depicted as pie charts. Central memory: CD45RA−CD45RO+CCR7+CD62L+; effector memory: CD45RA−CD45RO+CCR7−CD62L−; naive: CD45RA+CD45RO−CCR7+CD62L+; terminally differentiated: CD45RA+CD45RO−CCR7−CD62L−.
CRT exposure is associated with anticancer immune responses
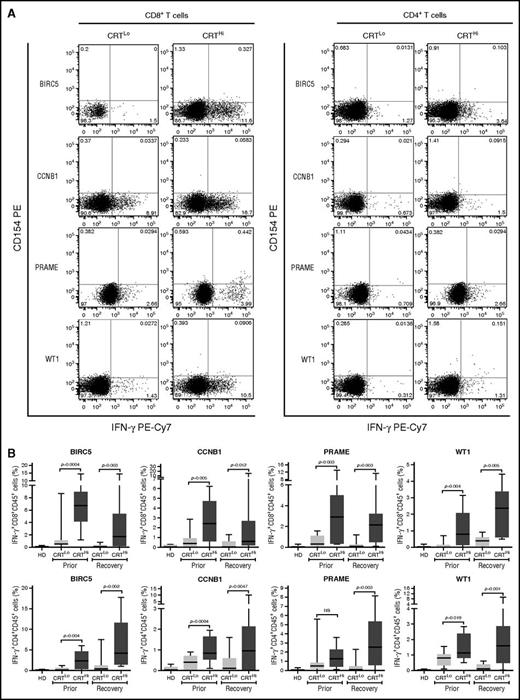
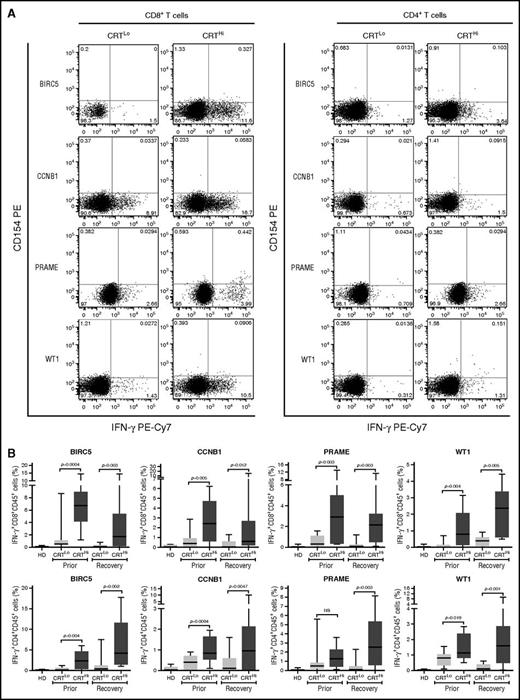
Leukemia-associated antigens (LAAs) have previously been shown to elicit tumor-specific immune responses in (at least some) patients.30 We therefore set out to investigate the relationship between CRT exposure and the frequency of leukemia-specific T cells in the peripheral blood of patients with AML prior to chemotherapy and upon the restoration of normal hematopoiesis. To this aim, we evaluated IFN-γ production by CD4+ and CD8+ T cells after exposing PBMCs from patients to peptide mixtures spanning several domains of BIRC5, CCNB1, PRAME and WT1 (supplemental Figure 6A). Both prior to the initiation of chemotherapy and at recovery, CRTHi patients with AML exhibited moderately increased amounts of LAA-specific CD4+ and CD8+ T lymphocytes in the peripheral blood as compared with their CRTLo counterparts (Figure 4A-B). Conversely, the percentage of circulating CD4+ and CD8+ T lymphocytes responding with IFN-γ secretion to a mixture of influenza-, cytomegalovirus-, and Epstein-Barr virus–derived epitopes did not differ between CRTHi and CRTLo patients (supplemental Figure 6B).
Antigen-specific immune responses in patients with AML exhibiting ecto-CRT+vs ecto-CRT−blasts. (A-B) Representative dot plots (A) and quantitative data (B) of IFN-γ–secreting CD45+CD3+CD4+ T cells and CD45+CD3+CD8+ T cells from 10 HD or 15 CRTHi vs 15 CRTLo patients with AML before induction chemotherapy (Prior, B) and at reestablishment of normal hematopoiesis (Recovery, A-B), upon exposure of the corresponding PBMCs to peptide mixture spanning BIRC5, CCNB1, PRAME, or WT1. Box plots: lower quartile, median, upper quartile; whiskers, minimum value, maximum value.
Antigen-specific immune responses in patients with AML exhibiting ecto-CRT+vs ecto-CRT−blasts. (A-B) Representative dot plots (A) and quantitative data (B) of IFN-γ–secreting CD45+CD3+CD4+ T cells and CD45+CD3+CD8+ T cells from 10 HD or 15 CRTHi vs 15 CRTLo patients with AML before induction chemotherapy (Prior, B) and at reestablishment of normal hematopoiesis (Recovery, A-B), upon exposure of the corresponding PBMCs to peptide mixture spanning BIRC5, CCNB1, PRAME, or WT1. Box plots: lower quartile, median, upper quartile; whiskers, minimum value, maximum value.
ICD-associated DAMPs correlate with improved disease outcome in patients with AML
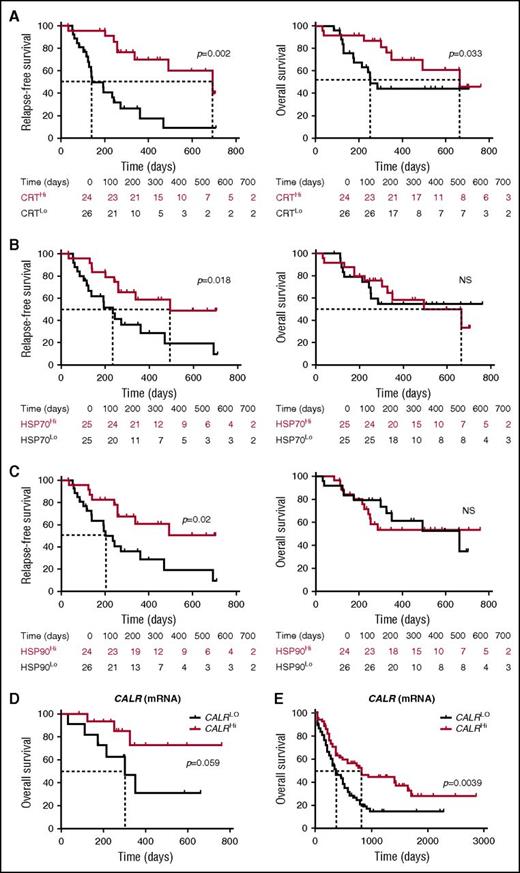
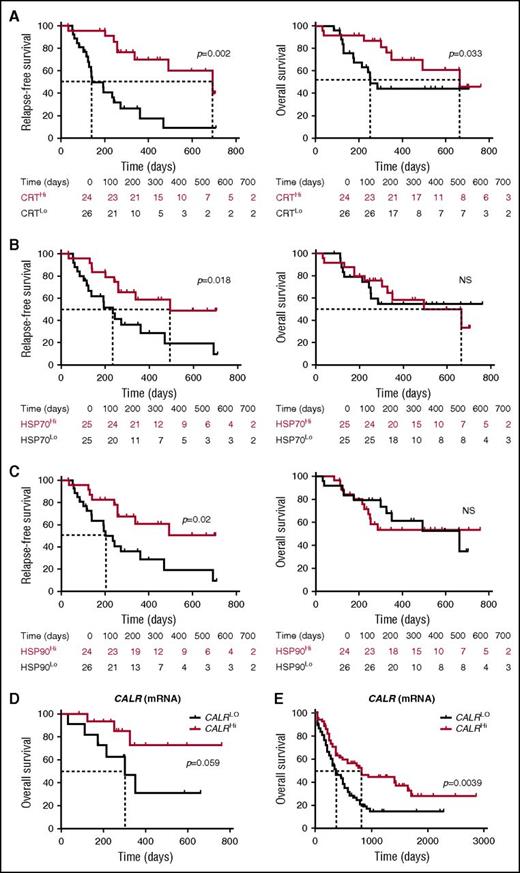
To evaluate the prognostic impact of ecto-CRT, we investigated RFS and OS upon stratifying the entire patient cohort based on median percentage of living (DAPI−) ecto-CRT+ blasts into a CRTHi (n = 24) and a CRTLo (n = 26) group. Importantly, CRTHi patients exhibited a significantly improved RFS (median: 692 vs 141 days; p = .002) and OS (median: 665 vs 252 days; P = .033) as compared with their CRTLo counterparts (Figure 5A). Using a similar median cutoff approach, we investigated RFS and OS in HSP70Hi (n = 25) vs HSP70Lo (n = 25) patients with AML, as well as in HSP90Hi (n = 24) vs HSP90Lo (n = 26) individuals. We found that both HSP70Hi and HSP90Hi patients exhibited an improved RFS as compared with their HSP70Lo (median: 493 vs 235 days; P = .018) and HSP90Lo counterparts (median: >700 vs 202 days; P = .02) (Figure 5B-C). However, neither the percentage of living (DAPI−) ecto-HSP70+ blasts nor that of living (DAPI−) ecto-HSP90+ blasts had a significant impact on OS in this cohort, although there was a tendency for improved OS among HSP70Hi patients (Figure 5B-C). Along similar lines, the circulating levels of HMGB1 measured prior to the initiation of chemotherapy did not influence disease outcome for these patients (supplemental Figure 7). Univariate Cox proportional hazards analysis confirmed that reduced percentages of living (DAPI−) ecto-CRT+, ecto-HSP70+, and ecto-HSP90+ blasts before induction chemotherapy (but not the circulating levels of HMGB1) were all associated with an increased risk for relapse (Table 2). However, this could not be confirmed on multivariate Cox proportional hazards analysis (Table 3), most likely owing to the limited number of patients.
Prognostic value of CRT, HSP70, and HP90 exposure in patients with AML. (A-C) RFS and OS among 50 patients with AML stratified in 2 groups based on median percentage of circulating ecto-CRT+ (A), ecto-HSP70+ (B), or ecto-HSP90+ (C) blasts measured prior to induction chemotherapy. Patients at risk are reported. The Wilcoxon test was employed to assess statistical significance. (D-E) Overall survival of 30 patients with AML from our cohort (D) and 173 patients with AML from the TCGA public database (E) stratified in 2 groups based on median CALR mRNA levels. Patients at risk are reported.
Prognostic value of CRT, HSP70, and HP90 exposure in patients with AML. (A-C) RFS and OS among 50 patients with AML stratified in 2 groups based on median percentage of circulating ecto-CRT+ (A), ecto-HSP70+ (B), or ecto-HSP90+ (C) blasts measured prior to induction chemotherapy. Patients at risk are reported. The Wilcoxon test was employed to assess statistical significance. (D-E) Overall survival of 30 patients with AML from our cohort (D) and 173 patients with AML from the TCGA public database (E) stratified in 2 groups based on median CALR mRNA levels. Patients at risk are reported.
As compared with their CRTLo counterparts, CRTHi patients exhibited reduced levels of multiple LAAs, including BIRC5, BMI1 proto-oncogene, polycomb ring finger, CCNB1, ELANE, HMMR; MOK; MPP1, PRAME, PRTN3, and WT1, in either a subsignificant or statistically significant manner (supplemental Figure 8A). Because LAA levels have been previously associated with improved disease outcome in patients with AML,31,32 we tested whether this would hold true in our cohort using the median cutoff approach. We failed to identify prognostic value (on both RFS and OS) for the expression levels of all the aforementioned LAAs (supplemental Figure 8B; Tables 2 and 3), suggesting that CRT exposure conveys superior prognostic information in this setting. It remains to be determined whether the decrease in LAA expression observed among CRTHi patients is a consequence of ecto-CRT–driven anticancer immune responses.
Finally, driven by recent results demonstrating that increased levels of the CALR mRNA in the tumor predict the response of patients with lung and ovarian cancer to chemotherapy with ICD inducers,33 we investigated the prognostic value of CALR mRNA levels in our patient cohort. We identified a trend for improved OS among patients with AML whose blasts contained higher-than-median (as compared with lower-than-median) CALR mRNA levels (P = .059) (Figure 5D). When we analyzed 173 patients from the TCGA database with the same median cutoff approach, high intratumoral CALR mRNA levels were strongly associated with improved OS (P = .0039) (Figure 5E). Unfortunately, ecto-CRT data are not available to investigate the prognostic impact of CRT exposure in this cohort. Taken together, these findings indicate that the spontaneous overexpression and exposure of CRT by malignant blasts conveys robust prognostic information for patients with AML.
Discussion
Throughout the past decade, our understanding of the mechanisms underlying the recognition and elimination of dying cancer cells by the immune system has considerably advanced.6,7 Nonetheless, little is known on the prognostic value of DAMPs and DAMP-associated processes in hematologic malignancies like AML, irrespective of the fact that these tumors are often treated with ICD inducers like anthracyclines and bortezomib.21 Here, we assessed the exposure and/or release of various DAMPs by malignant blasts in a prospective cohort of 50 patients with AML, before and after induction chemotherapy as well as upon the restoration of normal hematopoiesis. We found a rather heterogeneous proportion of blasts exposing CRT, HSP70, or HSP90 in patients (but not in healthy volunteers), regardless of chemotherapy (Figure 1). This observation suggests that, in the majority of patients with AML, malignant blasts experience chemotherapy-independent stress leading to the emission of danger signals, most likely as a consequence of malignant transformation itself (which imposes a considerable overload on most cellular processes, including protein synthesis and folding).34 Accordingly, we identified a robust correlation between CALR expression and the expression of 3 distinct genes involved in ER stress responses (ie, ATF4, HSPA5, and DDIT3) among 174 patients with AML from the TCGA database (Figure 1).
By combining transcriptomic studies and flow cytometry, we demonstrated that CRT exposure on malignant blasts correlates with the activation of a tumor-targeting TH1 immune response culminating in an increase in circulating tumor-specific IFN-γ-producing CD4+ and CD8+ cells (Figures 2-4). These results are in line with previous findings by us and others demonstrating that CRT exposure by neoplastic cells is associated with increased tumor infiltration by myeloid cells and effector memory CD8+ T cells in patients with non–small cell lung carcinoma,22 a high density of intratumoral T cells in patients with colorectal carcinoma,24 and signs of a tumor-specific immune response in patients with AML.26 Intriguingly, CRT exposure on malignant blasts also correlated with elevated amounts of circulating NK cells (Figure 3), perhaps reflecting the coexposure of HSP70 (which has previously been involved in clinically relevant antileukemia NK-cell responses).35,36
Importantly, exposure of CRT, HSP70, and HSP90 on malignant blasts (which was not influenced by disease subtype) was associated with improved RFS and decreased odds for relapse on univariate (but not multivariate) Cox proportional hazard analysis (Figure 5;,Tables 2-3). These findings were corroborated by the association between high CALR mRNA levels and improved OS in 173 patients with AML from the public TCGA database (Figure 5), as well as by previous results linking CRT expression and/or exposure on neoplastic cells with improved disease outcome in patients with neuroblastoma, non–small cell lung carcinoma, colorectal carcinoma, and ovarian carcinoma.22-24,33 Nonetheless, some studies that did monitor subcellular CRT localization failed to document a positive association between total CRT expression levels and favorable disease outcome.16,37,38 Intriguingly, we observed improved disease outcome among CRTHi patients irrespective of the chemotherapy employed for induction or consolidation, which included potentially immunosuppressive purine analogs (ie, cytarabine). These findings suggest that the chemotherapeutic regimens evaluated in this study are compatible with the activation of a tumor-targeting immune response.
Contrary to other studies,21 circulating HMGB1 levels did not correlate with RFS or OS in our patient cohort (supplemental Figure 7), but (1) were elevated in patients with AML as compared with healthy volunteers, and (2) returned to baseline along with the recovery of normal hematopoiesis (Figure 1). Thus, it is tempting to speculate that, in our patient cohort, serum HMGB1 may simply reflect the amount of circulating blasts (a proportion of which spontaneously releases HMGB1 in response to stress). This hypothesis remains to be formally addressed. Finally, we found that the expression level of multiple LAAs previously linked to disease outcome31,32 was devoid of prognostic value in our patients (supplemental Figure 8). Interestingly enough, however, all LAAs studied exhibited a (statistically significant or subsignificant) trend toward downregulation in CRTHi vs CRTLo patients (supplemental Figure 8). Whether such a decrease is a consequence of LAA-targeting immune responses supported by CRT exposure remains to be elucidated. It will also be interesting to investigate whether CRTHi patients with AML are more responsive to immunotherapy than their CRTLo counterparts, reflecting the increased immunogenicity of their blasts. If this were indeed the case, monitoring CRT exposure on malignant blasts could identify a subset of patients with AML that respond to some forms of immunotherapy (knowing that AML is generally refractory to multiple immunotherapeutics).39
Irrespective of these incognita, CRT stands out as a robust prognostic biomarker in patients with AML treated with chemotherapy. We surmise that a similar consideration may hold true for other hematological malignancies, and we plan to test this in the near future.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Anna Fialova for her valuable help with statistical analyses.
I.T. was supported by Student Research grant GAUK 682214 (Charles University, Prague, Czech Republic). The work of the Department of Immunology of Charles University is supported by Ministry of Health, Czech Republic-Conceptual Development of Research Organization (University Hospital Motol, Prague, Czech Republic, 00064203). O.K., L.G., and G.K. are supported by the Ligue contre le Cancer (équipe labelisée) and the European Research Council.
Authorship
Contribution: J.F., L.G., C.S., and R.S. planned the concept and design; J.F. and S.E.C. developed the methodology; J.F., I.T., M.H., L.K., I.M., S.V., J.K., and S.E.C. acquired the data; J.F., I.T., M.H., E.B., I.M., S.V., J.K., S.E.C., and I.C. analyzed and interpreted the data; J.F., I.C., O.K., G.K., L.G., C.S., and R.S. wrote, reviewed, and/or revised the manuscript; J.F. and R.S. were responsible for the study supervision.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Radek Spisek, Sotio, Jankovcova 1518/2, 170 00 Prague 7, Czech Republic; e-mail: spisek@sotio.com.
References
Author notes
J.F. and I.T. contributed equally to this study.