Key Points
Type I IFN therapies can cause a dose-dependent TMA.
Recombinant type I IFN therapies should be stopped at the earliest opportunity in patients who develop TMA.
Abstract
Many drugs have been reported to cause thrombotic microangiopathy (TMA), yet evidence supporting a direct association is often weak. In particular, TMA has been reported in association with recombinant type I interferon (IFN) therapies, with recent concern regarding the use of IFN in multiple sclerosis patients. However, a causal association has yet to be demonstrated. Here, we adopt a combined clinical and experimental approach to provide evidence of such an association between type I IFN and TMA. We show that the clinical phenotype of cases referred to a national center is uniformly consistent with a direct dose-dependent drug-induced TMA. We then show that dose-dependent microvascular disease is seen in a transgenic mouse model of IFN toxicity. This includes specific microvascular pathological changes seen in patient biopsies and is dependent on transcriptional activation of the IFN response through the type I interferon α/β receptor (IFNAR). Together our clinical and experimental findings provide evidence of a causal link between type I IFN and TMA. As such, recombinant type I IFN therapies should be stopped at the earliest stage in patients who develop this complication, with implications for risk mitigation.
Introduction
Thrombotic microangiopathy (TMA) syndromes are characterized by endothelial dysfunction, microangiopathic hemolytic anemia, and microvascular ischemia, with diverse etiologies that include drugs.1,2 Clinicians evaluating TMA patients must decide whether a particular drug is likely to have caused the disease. This difficult decision requires high-quality evidence, and recent work has highlighted the difficulty of attributing a causal relationship.1 TMA is typically a rare but serious adverse event that can occur after many years of treatment. As such, an association is unlikely to be detected in randomized controlled trial data.3 Therefore, for the large majority of drugs, causality with TMA is inferred from isolated case reports, without wider analyses of drug safety data or experimental evidence.1
This problem is exemplified by recombinant type I interferon (IFN) therapies. Recombinant IFN-α and IFN-β therapies act through the common type I IFN-α/β receptor (IFN-α receptor-1 [IFNAR]), and are widely used for the treatment of neoplastic, autoimmune and infectious diseases.4 Case reports have linked TMA to both IFN-α and IFN-β therapies, the main subclasses of type I IFN.5,6 Particular concern has been recently raised regarding IFN-β use in multiple sclerosis patients, where fatal cases of TMA have been observed.6,7 However, a causal role for IFN remains to be demonstrated, and alternative confounding etiologies such as other drugs, complement mutations, and Escherichia coli exposure have been suggested.6,8
Establishing causation in drug safety is a major challenge. Frameworks have been proposed to support evidence of a causal association between disease and environmental factors, the best known of which are the Bradford-Hill criteria.9,10 Such frameworks include a potential role for biological and experimental studies in establishing causation. As such, the demonstration of causality in drug safety benefits from a multifaceted approach to the adverse drug event, encompassing analyses of individual cases, drug safety data, and experimental evidence.10 Critically, such analyses require accurate description of the adverse drug event of interest. This is particularly relevant to the study of TMA because this is a pathophysiologically heterogeneous syndrome, with at least 9 primary TMA syndromes described.2 It is also important to establish whether an adverse event is caused by the drug’s active ingredient or by other drug components.11,12 For example, renal failure caused by intravenous immunoglobulin therapy is associated with the high sucrose content of the drug, rather than the immunoglobulin itself, with important implications for understanding the adverse event and managing risk.11
To address these questions, we performed a detailed clinical analysis of type I IFN–associated TMA cases presenting to a national TMA center to identify important features of the clinical phenotype of this complication. We provide experimental evidence that suggests that the IFN protein itself directly causes microvascular disease, using a transgenic model of type I IFN (IFN-α1) toxicity. We subsequently consider the potential implications of these findings for the safety of patients receiving recombinant type I IFN therapies.
Methods
Patient evaluation and drug safety data
Patients with multiple sclerosis who developed TMA with IFN-β therapy were referred to the national TMA center in Newcastle for further evaluation.13 The study was approved by Newcastle and North Tyneside 1 Research Ethics Committee (MREC/1/3/83). Individual patient IFN dose was determined from patient records and adjusted for weight (micrograms per kilogram). Dose-response was also further assessed through analysis of national spontaneous reporting data. Requests for safety data were submitted in the context of a registered patient safety audit (NHS Lothian DCNQIT417). Cases of IFN-associated TMA reported throughout the United Kingdom (UK) were identified through a request for spontaneous data submitted to the UK Medicines and Healthcare Products Regulatory Authority (MHRA). All spontaneously reported cases of TMA associated with recombinant IFN-β from 1999 to January 2016 to the MHRA were identified, using search criteria “hemolytic uremic syndrome,” “thrombotic thrombocytopenic purpura,” “thrombotic microangiopathy,” and “malignant hypertension.” These cases represent all TMA cases spontaneously reported using the UK “Yellow Card” spontaneous reporting scheme and UK cases reported in the published literature.
Immunohistochemical analysis
Paraffin-embedded renal biopsies from patients with IFN-β-associated TMA were cut into 5-µm sections, rehydrated, and boiled in sodium citrate buffer (pH 6.0). Sections were stained with mouse-anti-MxA (kind gift of Otto Haller, University of Freiburg, Freiburg im Breisgau, Germany; 1:400) followed by staining with EnVision G|2 System/AP Rabbit/Mouse (Dako) and counterstained with Mayer’s hematoxylin (Merck).
In vitro effects of type I IFN on endothelial cells
BEND.5 immortalized mouse brain endothelial cells were plated at 5 × 105 cells per well of a 6-well plate in 1 mL Dulbecco’s modified Eagle medium containing 10% fetal calf serum and incubated at 37°C with 5% CO2. Recombinant mouse or human IFN-α or IFN-β (R+D Systems: mouse IFN-αA, 12100-1; mouse IFN-β, 12400-1; human IFN-β1a, 11415-1; human IFN-α2, 11105-1) was added to culture media at concentrations of 0 to 104 U/mL and incubated for 24 hours. Total RNA was isolated from each well using QIAshredder and RNeasy (Qiagen), according to the manufacturer’s instructions. Each biological replicate was assayed for expression of Oas1A and Ifit1 and the housekeeping gene Hprt using Brilliant II SYBR master mix (Agilent Technologies). Thermocycling and data acquisition were performed using ABI Prism 7900HT Real-time PCR system. A Student t test was performed to compare relative gene expression at the highest IFN dose with no IFN. (GraphPad Prism, version 6.0d). For human endothelial cells (HUVECs), we accessed publically available microarray data from Interferome v. 2.0114 for HUVECs treated with IFN-α and IFN-β (recombinant protein from Schering-Plough at 1000 IU/mL).15 The fold-change for genes in IFN-α- and IFN-β-treated HUVECs were compared.
Analysis of microvasculature in a mouse model of type I IFN toxicity
The generation of transgenic mice (termed GFAP-IFN-α1) with astrocyte-targeted, brain-specific production of type I IFN has been described previously.16 Animal experiments were approved by University of Sydney Animal Ethics Committee (protocol number 5374). All animal experimental procedures were carried out in compliance with local procedures and guidelines. The microvasculature of nontransgenic littermate wild-type mice (WT), transgenic mice with low levels of astrocyte-derived IFN-α1 production (IFNLow), and high levels of IFN-α1 production (IFNHigh) was examined and quantified. Mice were perfused intracardially at 2 to 3 months of age, with ice-cold saline followed by 4% paraformaldehyde in phosphate-buffered saline (PBS; n = 8, each group). Brains were removed, postfixed overnight in the same fixative, dehydrated through graded alcohol solutions, and embedded in paraffin. For immunohistochemical detection of T cells and endothelial cells, sections were deparaffinized, rehydrated in graded alcohol, rinsed in PBS, and blocked for 1 hour at room temperature in PBS containing 1% bovine serum albumin. The sections were then incubated overnight at 4°C with primary antibody diluted in blocking buffer (CD3, Abcam rabbit monoclonal ab16669 and CD31, Abcam rabbit polyclonal ab28364). The sections were then washed in PBS and incubated with anti-rabbit avidin-biotinylated horseradish peroxidase complex (ABC kit; Vector, Burlingame, CA) used according to the manufacturer’s instructions. The total number of microvascular abnormalities was counted across 3 ×20 magnification fields per anatomical region, with counting performed blind to mouse genotype. One-way analysis of variance (ANOVA) was performed across groups for each anatomical region (GraphPad Prism, version 6.0d). For rescue experiments, IFNHigh mice were crossed to IFNAR−/− mice, which lack a functional type 1 IFN receptor.17
For RNA analysis, whole brain was removed and immediately snap-frozen in liquid nitrogen. Total RNA was extracted from the tissue samples using TriReagent (Sigma-Aldrich) performed according to the manufacturer’s instructions. The RNA concentration of the samples was determined by UV spectroscopy at 260 nm. For all probe sets, a fragment of the L32 gene was included and served as an internal loading control. The ribonuclease protection assays for IFN response genes (IRGs) were performed and analyzed as described previously,18,19 with autoradiographs quantified by densitometry using Image J software (n = 3 each group).
Vascular casting of IFN transgenic mice
Vascular casting of WT (n = 2) and IFNHigh mice (n = 6) was performed as previously described,20 in accordance with National Institutes of Health Guidelines for Care and Use of Laboratory Animals. Briefly, deeply anesthetized animals were perfused with artificial cerebrospinal fluid containing heparin, followed by 4% paraformaldehyde in PBS, and an injection of the resin Mercox (Ladd Research, Williston, VT). After resin curing, soft tissue was subsequently macerated followed by decalcification with 5% formic acid. Casts were washed, then dried by lyophilization, mounted on stubs, and sputter-coated with gold for routine scanning electron microscopy.
Results
IFN-β causes a direct drug-induced TMA
In view of the recent regulatory concerns regarding the potential association between type I IFN therapy and TMA in multiple sclerosis patients,6 we first performed a detailed analysis of the clinical features of IFN-β-associated TMA. The clinical phenotype of new and index6 cases of IFN-β-associated TMA in multiple sclerosis patients was reviewed by the UK national TMA center13 (n = 8, Table 1; Figure 1). Microangiopathic hemolytic anemia, renal failure, and severe hypertension were universally seen, and at least 1 other organ was affected, with prominent brain and cardiac involvement (Figure 1A-D). No patient received any other drugs associated with TMA,1 and no other confounding TMA triggers or other autoimmune diseases were identified.1,21-23 Detailed genetic evaluation, including exome sequencing, did not identify mutations in any gene associated with TMA. The final diagnostic evaluation by the national center was uniformly consistent with a chronic drug-induced TMA (Table 1; supplemental Table 1, available on the Blood Web site).1,24,25
IFN-β causes a direct drug-induced TMA in multiple sclerosis patients. Recombinant IFN-β therapy causes a microangiopathy affecting multiple organs in multiple sclerosis patients. (A-B) Magnetic resonance imaging brain scan shows multiple small new lesions consistent with recent microvascular ischemia, identified on diffusion-weighted sequences (white arrows). (C) Admission blood film showing fragmented red blood cells (black arrows). (D) Hematoxylin and eosin stain of renal biopsy from patient shows pathological microvascular changes with endothelial swelling, luminal narrowing, and trapped red blood cells (black arrow; bar represents 25 μm). (E) Patients who developed TMA, including patients from an independent cohort,7 received a higher weight-adjusted dose than unaffected multiple sclerosis patients treated with the same IFN-β preparation. *P < .001, Student t test. (F) All UK reports of TMA associated with IFN-β are associated with IFN-β1a dose >50 μg/wk, and 92% are associated with the highest available dose (n = 15 reports). (G-H) Evidence of activation of IFN response in renal biopsy of affected patient (H: MxA immunohistochemistry, red; bar represents 10 μm), with biopsy from patient with TMA not associated with IFN (G: genetic atypical hemolytic uremic syndrome [HUS]) for comparison.
IFN-β causes a direct drug-induced TMA in multiple sclerosis patients. Recombinant IFN-β therapy causes a microangiopathy affecting multiple organs in multiple sclerosis patients. (A-B) Magnetic resonance imaging brain scan shows multiple small new lesions consistent with recent microvascular ischemia, identified on diffusion-weighted sequences (white arrows). (C) Admission blood film showing fragmented red blood cells (black arrows). (D) Hematoxylin and eosin stain of renal biopsy from patient shows pathological microvascular changes with endothelial swelling, luminal narrowing, and trapped red blood cells (black arrow; bar represents 25 μm). (E) Patients who developed TMA, including patients from an independent cohort,7 received a higher weight-adjusted dose than unaffected multiple sclerosis patients treated with the same IFN-β preparation. *P < .001, Student t test. (F) All UK reports of TMA associated with IFN-β are associated with IFN-β1a dose >50 μg/wk, and 92% are associated with the highest available dose (n = 15 reports). (G-H) Evidence of activation of IFN response in renal biopsy of affected patient (H: MxA immunohistochemistry, red; bar represents 10 μm), with biopsy from patient with TMA not associated with IFN (G: genetic atypical hemolytic uremic syndrome [HUS]) for comparison.
Drug-induced TMAs can be caused by a direct toxic effect of the drug or via indirect immune-mediated mechanisms.1 Given that direct toxic reactions are typically dose dependent,24 we next examined the relationship between TMA and IFN dose. Affected patients were all in the bottom weight quartile, adjusting for age and sex.26 This observation of low weight was replicated in an independent case series.7 Therefore, patients who developed TMA received a significantly higher weight-adjusted IFN dose than those who did not develop the complication (Figure 1E; P < .001, Student t test). To confirm this dose dependence, we requested national spontaneous reporting data from the UK MHRA. This showed that all cases were associated with a dose of IFN-β in excess of 50 μg per week, with 92% of cases associated with the highest available dose (Figure 1F). Evidence of IFN bioactivity (Figure 1G-H) was detected in patient renal biopsies, and anti-IFN antibodies were not detected in any patient, consistent with a direct rather than immune-mediated effect of the drug. Taken together, these results suggest that IFN-β causes a toxic drug-induced TMA in multiple sclerosis patients.
TMA is observed with all type I IFN subtypes
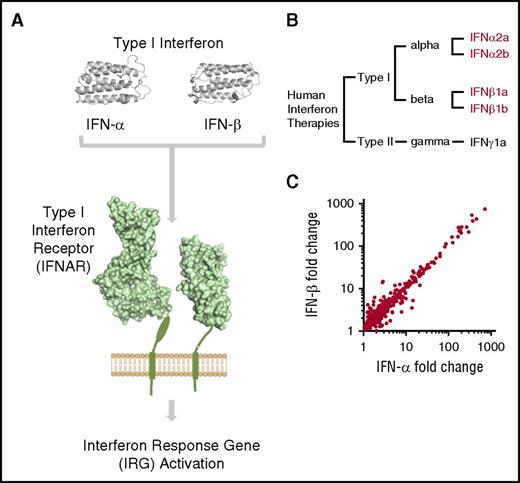
We next asked whether TMA was observed with all subtypes of recombinant type I IFN therapies in clinical use. The type I IFN family of proteins consists of a family of 13 α subtypes and a single major β subtype.27 These subtypes exhibit structural similarity and exert their biological activity via IFNAR (Figure 2A). Recombinant IFN-α therapies are used to treat a broad range of diseases such as chronic myeloid leukemia, hepatitis C, and melanoma. Systematic review of the literature shows that TMA has been reported as a complication of every subtype of recombinant type I IFN in clinical use: IFN-α2a, IFN-α2b, IFN-β1a, and IFN-β1b (Figure 2B; supplemental Figure 1).
TMA is associated with all type I IFN subtypes in clinical use. (A) An overview of type I IFN signaling. IFN-α and IFN-β act via the transmembrane IFNAR, leading to widespread upregulation of IRGs (three-dimensional protein models rendered using PyMOL Molecular Graphics System, version 1.8.2.2, Schrödinger LLC, with protein data obtained from the Protein Data Bank,43 http://www.rcsb.org). (B) Dendrogram of human recombinant IFN subtypes in clinical use. Recombinant type I IFN proteins associated with TMA highlighted in red (see supplemental Figure 1 for full details). (C) Comparison of the transcriptomic response of HUVECs to human IFN-α and IFN-β.14 The transcriptional response of HUVECs to both type I IFN subtypes is highly correlated (r = 0.97; genes with fold-change >1 shown).
TMA is associated with all type I IFN subtypes in clinical use. (A) An overview of type I IFN signaling. IFN-α and IFN-β act via the transmembrane IFNAR, leading to widespread upregulation of IRGs (three-dimensional protein models rendered using PyMOL Molecular Graphics System, version 1.8.2.2, Schrödinger LLC, with protein data obtained from the Protein Data Bank,43 http://www.rcsb.org). (B) Dendrogram of human recombinant IFN subtypes in clinical use. Recombinant type I IFN proteins associated with TMA highlighted in red (see supplemental Figure 1 for full details). (C) Comparison of the transcriptomic response of HUVECs to human IFN-α and IFN-β.14 The transcriptional response of HUVECs to both type I IFN subtypes is highly correlated (r = 0.97; genes with fold-change >1 shown).
We next compared the detailed clinical phenotype described here of IFN-β-associated TMA with a comparably detailed analysis of a series of IFN-α-associated TMA, analyzed at a specialized center5 (n = 8 both groups). A similar clinical phenotype is observed in both case series (supplemental Table 2). Specifically, IFN-α and IFN-β TMA were associated with long-term therapy at high doses.5 Both groups displayed severe hypertension, predominant involvement of the renal microvasculature, moderate thrombocytopenia, and a poor renal outcome. All published cases are summarized in supplemental Tables 3 and 4 and confirm that this clinical picture is seen in the majority of patients. Furthermore, the transcriptomic response of HUVECs to both human IFN-α and IFN-β are highly correlated (Figure 2C; r = 0.97).15 Therefore, TMA can occur with all subtypes of recombinant type I IFN therapies, and these subtypes exert an almost identical effect on endothelial cells.
TMA is associated with “interferonopathic” disease
An activated type I IFN response can be induced by recombinant protein therapies but can also occur spontaneously in patients in whom endogenous levels of type I IFN are pathologically elevated. These “interferonopathic” diseases include monogenic disorders of IFN dysregulation, as well as more common autoimmune diseases such as systemic lupus erythematosus (SLE).28,29 We reviewed pathological findings from patients with different diseases where transcriptomic evidence of an IFN signature has been reported and identified an association with histopathological evidence of TMA, which is exceptionally rare as a spontaneous disease30 (supplemental Figure 1). Therefore, TMA is observed in states of elevated endogenous and exogenous type I IFN in humans.
Type I IFN signaling is species specific, limiting interpretation of preclinical toxicity tests
Given this clinical evidence that TMA is a manifestation of IFN toxicity, we next sought to provide experimental evidence that the recombinant protein itself can cause the small vessel disease observed in the patients described here. To address whether IFN can directly cause microangiopathic disease in rodent models, we first evaluated available preclinical toxicity data used in the clinical development of both recombinant IFN-α and -β therapies.31,32 No reports of microvascular toxicity were recorded in extensive preclinical testing of human recombinant protein in rodents. However, type I IFN signaling is highly species specific, and human IFN-α and -β do not cause upregulation of IRGs in mouse endothelial cells (Figure 3A-B). Therefore, preclinical rodent toxicity models cannot be used to evaluate whether IFN causes endothelial toxicity.31,32
Type I IFN signaling is species specific, limiting interpretation of cross-species preclinical toxicity models. Type I IFN proteins (-α and -β) act through a common receptor (IFNAR), leading to activation of downstream IRGs. (A) Preclinical toxicity testing of human recombinant type I IFN utilizes cross-species testing. Human recombinant type I IFN does not elicit a downstream IFN response in mouse endothelial cells, as measured by quantitative PCR for type I IRGs in BEND.5 brain endothelial cell line. Despite extensive preclinical testing (355 mice tested with human IFN-α, 347 mice tested with human IFN-β), no reports of small vessel toxicity were observed in publically available preclinical tests for human recombinant type I IFN therapies tested in rodents.31,32 (B) In contrast, dose-dependent upregulation of IRGs is observed in mouse endothelial cells exposed to same-species recombinant type I IFN in vitro (data represent mean ± standard error of the mean [SEM]; *P < .05, Student t test compared with no IFN; n = 3 experiments, IFN-α shown).
Type I IFN signaling is species specific, limiting interpretation of cross-species preclinical toxicity models. Type I IFN proteins (-α and -β) act through a common receptor (IFNAR), leading to activation of downstream IRGs. (A) Preclinical toxicity testing of human recombinant type I IFN utilizes cross-species testing. Human recombinant type I IFN does not elicit a downstream IFN response in mouse endothelial cells, as measured by quantitative PCR for type I IRGs in BEND.5 brain endothelial cell line. Despite extensive preclinical testing (355 mice tested with human IFN-α, 347 mice tested with human IFN-β), no reports of small vessel toxicity were observed in publically available preclinical tests for human recombinant type I IFN therapies tested in rodents.31,32 (B) In contrast, dose-dependent upregulation of IRGs is observed in mouse endothelial cells exposed to same-species recombinant type I IFN in vitro (data represent mean ± standard error of the mean [SEM]; *P < .05, Student t test compared with no IFN; n = 3 experiments, IFN-α shown).
Transgenic overexpression of type I IFN causes a spectrum of microangiopathic disease, including changes seen in patient biopsies
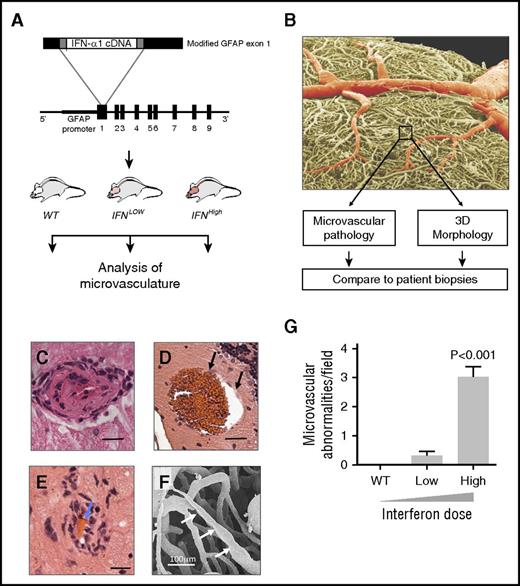
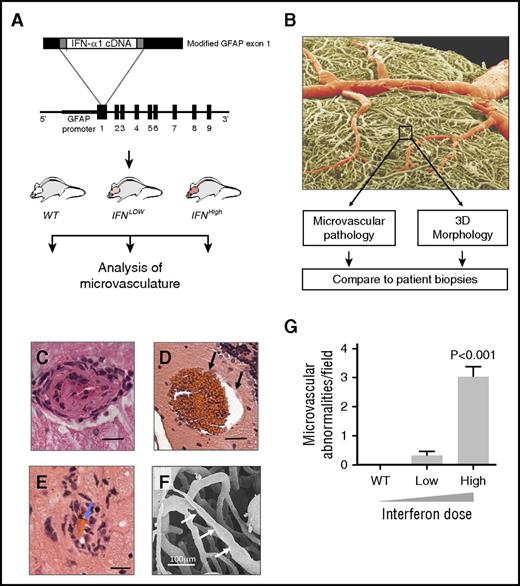
To overcome the problem of species disparity of IFN action, we used a species-matched transgenic mouse model to evaluate the effects of chronic type I IFN exposure on small blood vessels. In our model, type I IFN (IFN-α1) is transgenically produced in the brain at zero (WT), low (IFNLow), or high (IFNHigh) levels, leading to focal graded activation of the IFN response via IFNAR (Figure 4A).16 Quantitative histopathological analyses identified a dose-dependent spectrum of microvascular disease, including endothelial hypertrophy and intimal thickening, microaneurysms, and perivascular inflammatory infiltrates (Figures 4C-F, 5, and 6). This microangiopathy was observed across anatomical regions and was dose dependent (Figures 4G and 6D; P < .001, 1-way ANOVA).
Type I IFN causes a dose-dependent microangiopathy. (A) Overview of experimental design (see text), with structure of the fusion gene used to generate the GFAP-IFN-α transgenic mice. (B) Scanning electron micrograph of IFNHigh vascular cast, colored to show larger vessels (red) and microvasculature (box, yellow). (C-E) Hematoxylin and eosin sections of mice with maximal IFN overexpression show a spectrum of dose-dependent microangiopathic pathology, including endothelial hypertrophy and intimal thickening (C), microaneurysms (black arrows, D), and perivascular inflammatory cell infiltration and red bloods cells within narrowed lumen (blue arrows, E). Bars represent 20 μm. (F) Scanning electron micrograph of small vessel cast showing variation in microvessel caliber (white arrows). (G) Microvascular pathology in IFN-overexpressing mice is dose dependent. WT, wild type, no transgenic overexpression; IFNLow, transgenic line with low IFN overexpression; IFNHigh, transgenic line with high IFN overexpression (n = 8 mice per group). Data represent mean ± SEM; 1-way ANOVA, P < .001.
Type I IFN causes a dose-dependent microangiopathy. (A) Overview of experimental design (see text), with structure of the fusion gene used to generate the GFAP-IFN-α transgenic mice. (B) Scanning electron micrograph of IFNHigh vascular cast, colored to show larger vessels (red) and microvasculature (box, yellow). (C-E) Hematoxylin and eosin sections of mice with maximal IFN overexpression show a spectrum of dose-dependent microangiopathic pathology, including endothelial hypertrophy and intimal thickening (C), microaneurysms (black arrows, D), and perivascular inflammatory cell infiltration and red bloods cells within narrowed lumen (blue arrows, E). Bars represent 20 μm. (F) Scanning electron micrograph of small vessel cast showing variation in microvessel caliber (white arrows). (G) Microvascular pathology in IFN-overexpressing mice is dose dependent. WT, wild type, no transgenic overexpression; IFNLow, transgenic line with low IFN overexpression; IFNHigh, transgenic line with high IFN overexpression (n = 8 mice per group). Data represent mean ± SEM; 1-way ANOVA, P < .001.
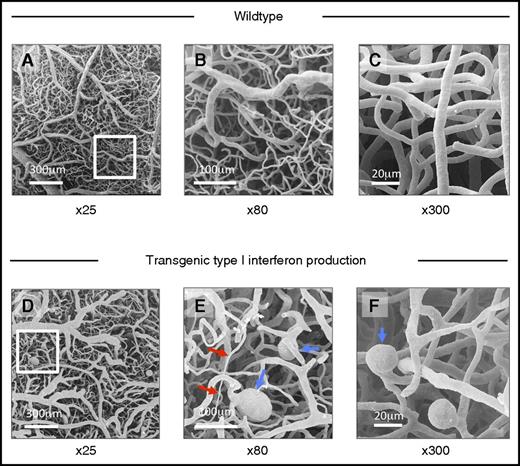
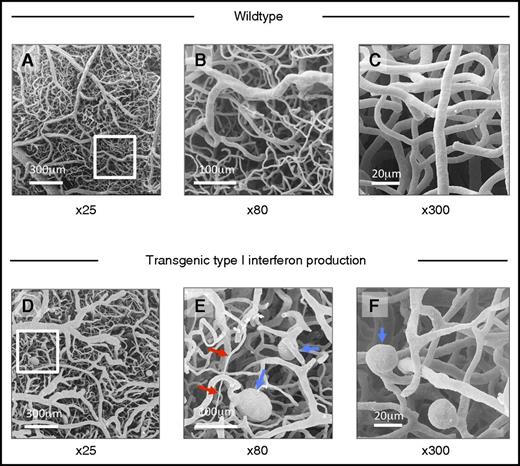
Abnormal morphology of cerebral microvascular casts of IFNHigh mice demonstrated by scanning electron microscopy. (A-C) Scanning electron micrograph of cerebral microvascular casts of wild-type mice at low (×25), medium (×80), and high power (×300). (D-F) Transgenic type I IFN production: Abnormal microvascular morphology in IFNHigh mice, with variations in microvessel caliber (red arrows), ectatic vessels, and microaneurysms (blue arrows). Such microvascular abnormalities were observed in all IFNHigh mice with highest levels of brain-restricted IFN-α1 production (n = 6) but not in WT mice.
Abnormal morphology of cerebral microvascular casts of IFNHigh mice demonstrated by scanning electron microscopy. (A-C) Scanning electron micrograph of cerebral microvascular casts of wild-type mice at low (×25), medium (×80), and high power (×300). (D-F) Transgenic type I IFN production: Abnormal microvascular morphology in IFNHigh mice, with variations in microvessel caliber (red arrows), ectatic vessels, and microaneurysms (blue arrows). Such microvascular abnormalities were observed in all IFNHigh mice with highest levels of brain-restricted IFN-α1 production (n = 6) but not in WT mice.
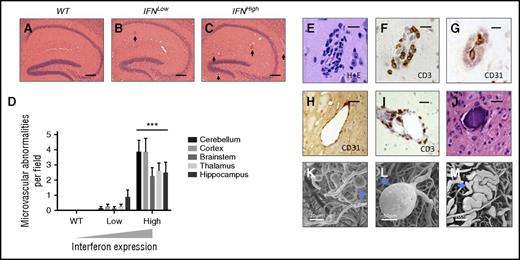
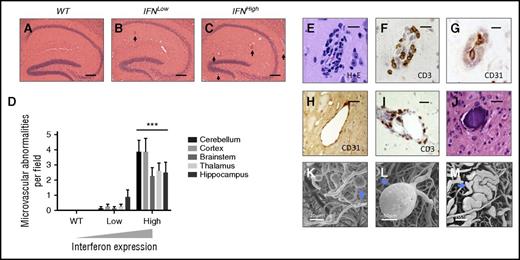
Spectrum of microvascular abnormalities observed in a focal model of type I IFN toxicity. A spectrum of microvascular disease is observed in mice with transgenic production type I IFN in the brain, across all anatomical regions. (A-C) Dose-dependent microvascular pathology in the hippocampus of transgenic mice with zero, low, and high levels of IFN overexpression (hematoxylin/eosin, abnormal small blood vessel highlighted by black arrow). (D) Dose-dependent microvascular pathology was observed across all major brain regions. ***P < .01, 1-way ANOVA for each region; n = 8 each group. (E-G) Perivascular inflammatory cell infiltration: hematoxylin/eosin (H+E), CD3, and CD31 immunohistochemistry shows small blood vessels with T-cell infiltration (CD3) and narrowing of lumen (CD31 endothelial marker). (H) Ectatic small vessel. (I) Perivascular infiltration by T cells (CD3, brown). (J) Intravascular calcification; bars represent 20 μm (E-G) and 40 μm (H, I). Scanning electron microscopy of microvascular cast shows multiple small capillary microaneurysms (K, blue arrow), large microaneurysm (L), and ectatic microvessel (M, blue arrow).
Spectrum of microvascular abnormalities observed in a focal model of type I IFN toxicity. A spectrum of microvascular disease is observed in mice with transgenic production type I IFN in the brain, across all anatomical regions. (A-C) Dose-dependent microvascular pathology in the hippocampus of transgenic mice with zero, low, and high levels of IFN overexpression (hematoxylin/eosin, abnormal small blood vessel highlighted by black arrow). (D) Dose-dependent microvascular pathology was observed across all major brain regions. ***P < .01, 1-way ANOVA for each region; n = 8 each group. (E-G) Perivascular inflammatory cell infiltration: hematoxylin/eosin (H+E), CD3, and CD31 immunohistochemistry shows small blood vessels with T-cell infiltration (CD3) and narrowing of lumen (CD31 endothelial marker). (H) Ectatic small vessel. (I) Perivascular infiltration by T cells (CD3, brown). (J) Intravascular calcification; bars represent 20 μm (E-G) and 40 μm (H, I). Scanning electron microscopy of microvascular cast shows multiple small capillary microaneurysms (K, blue arrow), large microaneurysm (L), and ectatic microvessel (M, blue arrow).
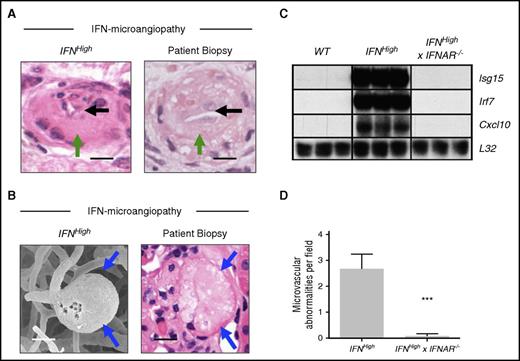
The three-dimensional morphology of small blood vessels is difficult to assess using conventional histopathological techniques; therefore, we performed scanning electron microscope analysis of microvascular casts. This confirmed widespread microvascular abnormalities in the brain, with prominent variations in microvascular caliber including luminal stenosis and occlusions, ectasia, and microaneurysms (Figures 5 and 6K-M). These abnormalities were not seen in WT mice. This dose-dependent type I IFN-associated microangiopathy included pathological microvascular abnormalities seen in the biopsies of the patients described previously, such as endothelial hyperplasia, luminal occlusion, and microaneurysm formation (Figure 7A-B).
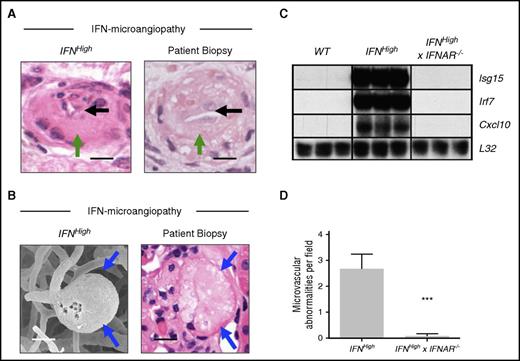
Specific pathological features of IFN-associated microangiopathy are seen in patient renal biopsy material and are mediated through the type I IFN receptor. (A-B) Microvascular pathology identified in this model includes specific pathological abnormalities such as luminal narrowing (black arrows), endothelial hyperplasia (green arrows), and microaneurysm formation (blue arrows), which were seen in the biopsies of the patients described previously. Bars represent 20 μm. (C) Upregulation of IRGs (Isg15, Irf7, Cxcl10) was observed in IFNHigh transgenic mice and absent in mice that lack the type I IFN receptor (IFNHigh × IFNAR−/−). Total RNA was extracted from the brain of WT and transgenic mice and analyzed by ribonuclease protection assay and visualized by autoradiography (n = 3 per group). (D) Rescue of microvascular pathology in IFNHigh mice that lack the type I IFN receptor (IFNHigh× IFNAR−/−). Data represent mean ± SEM; ***P < .001, Student t test compared with IFNHigh; n = 8 regions.
Specific pathological features of IFN-associated microangiopathy are seen in patient renal biopsy material and are mediated through the type I IFN receptor. (A-B) Microvascular pathology identified in this model includes specific pathological abnormalities such as luminal narrowing (black arrows), endothelial hyperplasia (green arrows), and microaneurysm formation (blue arrows), which were seen in the biopsies of the patients described previously. Bars represent 20 μm. (C) Upregulation of IRGs (Isg15, Irf7, Cxcl10) was observed in IFNHigh transgenic mice and absent in mice that lack the type I IFN receptor (IFNHigh × IFNAR−/−). Total RNA was extracted from the brain of WT and transgenic mice and analyzed by ribonuclease protection assay and visualized by autoradiography (n = 3 per group). (D) Rescue of microvascular pathology in IFNHigh mice that lack the type I IFN receptor (IFNHigh× IFNAR−/−). Data represent mean ± SEM; ***P < .001, Student t test compared with IFNHigh; n = 8 regions.
To confirm this microvascular pathology is caused by activation of the IFN response through IFNAR, we crossed IFNHigh mice with mice that were null for the type I IFN receptor,17 generating IFNHigh× IFNAR−/− mice. Both upregulation of IRGs and microvascular disease were absent in IFNHigh× IFNAR−/− mice, confirming a critical role of IFNAR in mediating disease (Figure 7C-D).
Taken together, these data suggest that type I IFN therapies cause a dose-dependent TMA and the IFN protein itself can cause microangiopathic disease.
Discussion
To date, unambiguous evidence of a causal association between type I IFN therapies and TMA has been lacking. This lack of evidence, together with a limited understanding of the serious adverse event, has hampered risk mitigation measures for these drugs and makes the clinical decision as to whether to stop IFN treatment in patients with TMA difficult. TMA is a particularly challenging adverse drug event to study because it is rare and may occur after prolonged exposure to the drug. As such, randomized clinical trials are not powered to identify a causal association.3,33 The detection of rare or delayed adverse events that occur outside trials typically relies on analysis of case reports and signal detection through spontaneous reporting data.3 This approach is subject to bias and confounding, and therefore, even if an association is identified, a causal relationship cannot be inferred. A number of conceptual frameworks have been proposed when considering how to infer causality from observational clinical data.10,34 Some of these frameworks, such as the well-known Bradford-Hill criteria,10 suggest that experimental evidence can provide an important role in establishing causation by demonstrating biological plausibility and direct evidence of causation in model systems. Our analyses of clinical, drug safety, and experimental data satisfy the Bradford-Hill criteria and therefore support a causal link between type I IFN therapy and TMA (supplemental Table 5).
Careful definition of the adverse event of interest is central to the study of drug side effects.9 This is of particular relevance here because many cases of IFN-associated TMA have been reported using diverse terms including HUS,7 thrombotic thrombocytopenic purpura (TTP),35 or malignant hypertension,35,36 each implying a different pathophysiological process. It is therefore an important first step to define the clinical syndrome through a center with expertise in the evaluation of this complex condition. Our analyses of cases suggest that IFN-β therapy is associated with a direct drug-induced TMA.2 Direct drug-induced TMAs, such as those induced by vascular endothelial growth factor inhibitors, are characterized by a gradual onset, exposure to high drug dose and absence of immunogenicity.1,2,24 This phenotype is consistently reproduced in our cohort here. Three lines of evidence suggest that IFN-β-associated TMA is associated with high dose therapy. First, patients who developed the complication received nearly twice the weight-adjusted dose than patients who did not develop the complication. Second, our data show that all cases of TMA spontaneously reported in the United Kingdom were associated with doses >50 μg/wk. Third, our experimental model recapitulates a striking dose dependence of endothelial disease.
Although our primary focus here has been to address recent ongoing concerns regarding recombinant IFN-β use in multiple sclerosis patients, our wider analyses suggest that TMA is associated with all subtypes of recombinant type I IFN in clinical use. Comparison of case series evaluated at centers with TMA expertise suggests that TMA with IFN-α and IFN-β therapy display a similar clinical phenotype. In particular, both are associated with prolonged exposure to high doses of IFN, with chronic changes observed on biopsy. This is characterized by panendothelial disease with prominent renal, cerebral, and cardiac involvement that may evolve over months. Further review of the published literature suggests that this clinical phenotype is consistently observed in almost all reported IFN-β TMA cases and most IFN-α TMA cases. Some patients with IFN-α TMA can occasionally present with a broader phenotype more suggestive of TTP, in particular patients with underlying hematological malignancies.5 Future deep phenotypic study of this adverse event across centers will be important to determine the spectrum and true incidence of this disease, as well as the influence of underlying morbidity on clinical presentation. We propose that the most appropriate working descriptive term for this complication is “type I interferon-associated thrombotic microangiopathy.”
We have tested the hypothesis that type I IFN itself can directly cause microvascular disease. This hypothesis cannot be evaluated in preclinical rodent models because of the species specificity of type I IFN signaling. We have therefore studied 2 complementary “species-matched” states of elevated type I IFN in mice and humans. First, our mouse model here provides experimental advantage over preclinical rodent models, through species matching of IFN, and allows evaluation of the chronic effects of IFN exposure. Analysis of the microvasculature of this model demonstrates that type I IFN causes a spectrum of microangiopathy, with features that overlap with microvascular disease observed in the patients we describe. Second, we show that TMA, which is exceptionally rare as a spontaneous disease,30 is consistently observed across a broad spectrum of human “interferonopathic” diseases. In these disease states, IFN levels are spontaneously elevated in the context of activated innate immunity.27 As such, they might be considered by the Bradford-Hill criteria to represent an “analogous” system of human IFN toxicity. These diseases include rare genetic disorders of IFN dysregulation4,27,28 and more common autoimmune disorders such as SLE.37 The precise subtypes of type I IFN that are elevated in these disease states are not yet fully described, but activation of the IFN response downstream of IFNAR is typically observed.28 Microangiopathy is a major cause of organ damage in these patients (supplemental Figure 1).38,39 For example, 25% of patients with SLE affecting the kidney have evidence of TMA on renal biopsy,40 and microangiopathy is a dominant brain pathology in patients with SLE.41 Therefore, microangiopathic disease is consistently seen in mouse and human states of aberrantly elevated type I IFN. It is notable that in both patients and the mouse model we describe, microangiopathy is the dominant pathological feature, with microvascular thrombosis a less prominent feature. The lack of thrombosis on biopsy in many TMAs has resulted in a recent call for a change in nomenclature from TMA to microangiopathy with or without thrombosis to reflect this incongruity.42
These findings are relevant to clinicians who must decide whether to stop recombinant type I IFN therapy in patients who develop TMA. Our data suggest a causal association, and therefore, the drug should be stopped at the earliest opportunity given the serious ensuing morbidity. Furthermore, our findings are of relevance to risk mitigation strategies because they raise the possibility of stratification and monitoring. In particular, detailed retrospective analysis of clinical features and biopsies of cases suggests that type I IFN causes a chronic TMA, which evolves over weeks to months, with a detectable prodrome (supplemental Table 6). At present, renal function monitoring and blood pressure monitoring are not mandated for patients receiving type I IFN therapies, and none of the patients described here were actively monitored for this complication. Therefore, despite a potential window of opportunity for early recognition, all patients presented at a late stage to emergency or critical care facilities. This late presentation is characterized by fulminant disease and organ failure, sometimes with fatal consequences.
Additional blood pressure and renal function monitoring for TMA have subsequently been introduced across Scotland following concerns about the severity and incidence of this complication in multiple sclerosis patients, where an incidence of ∼1:1000 patient-years has been observed in patients treated with high-dose IFN-β6 (supplemental Figure 2). This monitoring has led to prompt cessation of IFN-β in patients who have developed severe/accelerated hypertension associated with mild renal dysfunction. Since the implementation of targeted monitoring, no patients have developed fulminant organ failure because of IFN-associated TMA (supplemental Figure 2B). We suggest that further consideration should be given to risk mitigation measures for this serious adverse drug event.
In summary, we have shown that type I IFN therapies cause a direct dose-dependent TMA and the IFN protein itself can directly damage small blood vessels. The chronic and dose-dependent nature of this complication identifies an opportunity to reduce risk to the large numbers of patients exposed to these drugs. These findings should facilitate and stimulate definitive and rigorous pharmacoepidemiological study of this serious adverse event, with an emphasis on quantification of risk and the potential for risk mitigation across the available type I IFN therapies. In the meantime, clinicians should be aware of this association when assessing patients and stop recombinant type I IFN therapies at the earliest stage in patients who develop TMA.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank neurology consultants and nurses in all major Scottish multiple sclerosis centers and the MHRA for providing data as part of safety audit. The authors also thank James Ironside for advice on neuropathology and Tim Goodship and Martin Reijns for discussions on the manuscript.
D.P.J.H. and D.K. were supported by the Wellcome Trust (WT101153MA) (D.P.J.H.) and the Academy of Medical Sciences. C.G. was supported by a grant from the Deutsche Forschungsgemeinschaft (KFO 249/GU1212/1-1 and GU1212/1-2). A.P.J. was supported by the Medical Research Council. I.L.C. was supported by the National Health Medical Research Council (project grant 512407) and the National Institutes of Health, National Institute of Mental Health (grant MH47680).
Authorship
Contribution: All authors contributed to writing and editing the manuscript; D.P.J.H., B.W., J.O., and S.C. registered and analyzed audit with additional input from N.S., D.P.G., and D.K.; D.P.J.H. and D.K. collated clinical data for patients; C.B., C.G., Y.S.K., and H.B. performed histopathological analyses of human renal biopsy specimens; R.C. performed genetic analyses and exome sequencing; O.F., M.B., and D.K. were responsible for clinical care and evaluation of inpatients; I.L.C. generated transgenic mice; A.J. and D.P.J.H. analyzed neuropathology of transgenic mice; I.L.C., T.K., and E.P.M. performed electron microscopic analysis of microvasculature of transgenic mice; J.W. analyzed toxicity reports; S.M. performed in vitro endothelial cell culture experiments; and D.P.J.H., S.C., and S.J.W.E. performed pharmacoepidemiological analyses.
Conflict-of-interest disclosure: D.K. and D.P.J.H. are supported by the Wellcome Trust. B.W. is a principal investigator in clinical trials supported by Novartis, Sanofi-Aventis, and Biogen Idec and receives honoraria for advisory work from Merck, Biogen Idec, Novartis, and Bayer. D.K. received honoraria for consultancy work from Alexion Pharmaceuticals. D.P.G. received honoraria for consultancy from Alexion, Novartis, and Otsuka. T.K. is currently employed by Novartis (experimental work here performed prior to this). J.O. received honoraria for consultancy work and travel grants from Novartis, Teva UK Ltd., Biogen Idec, and Merck . N.S. received honoraria and/or research and/or educational support from Merck Serono, Biogen Idec, Teva, GSK, and Novartis. S.C. received honoraria for consultancy work from Merck Serono, Biogen Idec, and Novartis and research and/or educational support from Merck, Novartis, and Biogen Idec. The remaining authors declare no competing financial interests.
Correspondence: David P. J. Hunt, MRC Human Genetics Unit, Institute of Genetics and Molecular Medicine, University of Edinburgh, Crewe Rd, Edinburgh EH4 2XU, United Kingdom; e-mail: david.hunt@igmm.ed.ac.uk.

![Figure 1. IFN-β causes a direct drug-induced TMA in multiple sclerosis patients. Recombinant IFN-β therapy causes a microangiopathy affecting multiple organs in multiple sclerosis patients. (A-B) Magnetic resonance imaging brain scan shows multiple small new lesions consistent with recent microvascular ischemia, identified on diffusion-weighted sequences (white arrows). (C) Admission blood film showing fragmented red blood cells (black arrows). (D) Hematoxylin and eosin stain of renal biopsy from patient shows pathological microvascular changes with endothelial swelling, luminal narrowing, and trapped red blood cells (black arrow; bar represents 25 μm). (E) Patients who developed TMA, including patients from an independent cohort,7 received a higher weight-adjusted dose than unaffected multiple sclerosis patients treated with the same IFN-β preparation. *P < .001, Student t test. (F) All UK reports of TMA associated with IFN-β are associated with IFN-β1a dose >50 μg/wk, and 92% are associated with the highest available dose (n = 15 reports). (G-H) Evidence of activation of IFN response in renal biopsy of affected patient (H: MxA immunohistochemistry, red; bar represents 10 μm), with biopsy from patient with TMA not associated with IFN (G: genetic atypical hemolytic uremic syndrome [HUS]) for comparison.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/128/24/10.1182_blood-2016-05-715987/4/m_blood715987f1.jpeg?Expires=1765965109&Signature=uxgXEGJ9OdCc13IoUYgZAZXhVEjGdgtTcIKEH15zo~fMB7e1HAiWCMSlEn29Q~o4eqwXCr0Vn~cPbjji62AGgnNAyOwAZ8LRguVsJndUgGeaa7ncQaLbNOegZf3sJsqie7dfRdr9VKLnO1k6873fVOI7Mr1Dqp~XMxGNll0DncX9Axo4I3NgIemisnryQXcfKFeyoM48BqTFntWJEWd0kvhqog1OMvVGSXjTVZoVEfCQ3BmeEya~JxN4-VjvezeD-pjDoAPUtCp-2tBQOLD5GqoiJHefDJsR9JteoWd8b1WSrRnoHr1jdj48BVOKbGclIkAGYzmtnlt4lrT0w-UXRg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

![Figure 3. Type I IFN signaling is species specific, limiting interpretation of cross-species preclinical toxicity models. Type I IFN proteins (-α and -β) act through a common receptor (IFNAR), leading to activation of downstream IRGs. (A) Preclinical toxicity testing of human recombinant type I IFN utilizes cross-species testing. Human recombinant type I IFN does not elicit a downstream IFN response in mouse endothelial cells, as measured by quantitative PCR for type I IRGs in BEND.5 brain endothelial cell line. Despite extensive preclinical testing (355 mice tested with human IFN-α, 347 mice tested with human IFN-β), no reports of small vessel toxicity were observed in publically available preclinical tests for human recombinant type I IFN therapies tested in rodents.31,32 (B) In contrast, dose-dependent upregulation of IRGs is observed in mouse endothelial cells exposed to same-species recombinant type I IFN in vitro (data represent mean ± standard error of the mean [SEM]; *P < .05, Student t test compared with no IFN; n = 3 experiments, IFN-α shown).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/128/24/10.1182_blood-2016-05-715987/4/m_blood715987f3.jpeg?Expires=1765965109&Signature=v5GTRREgeqcEjs4DcRSNqiwGlyIBg1f54w3LaC~5SsE4n7-3fmlMfN3mPTTfcYkT68dXmiYy~q9quH9CjOL4wQRbyuAqc1KcgCWGh4o4iJoJ~xGyy6FOVozOyFdjnuUqD3dAzPsqPYzVC8GGVzltd6FiR3qDgj82xQeSt0BVYasO8ZjhWGTVgcn4WhsCjYtkbYzLapIUX0ods47wmFekXcSRYIiFU4R47rKpcreBsF7VkNiyMKNxtR1Z-P0iDSQ12~ReOu5zVXuayyYYvFe8K7XMZF8BjmwQflcL-MmhfY9UYqFBh94J0iZ9CMENb2l1NoxRQyCWyuEz8Z0u4S1MvQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

![Figure 1. IFN-β causes a direct drug-induced TMA in multiple sclerosis patients. Recombinant IFN-β therapy causes a microangiopathy affecting multiple organs in multiple sclerosis patients. (A-B) Magnetic resonance imaging brain scan shows multiple small new lesions consistent with recent microvascular ischemia, identified on diffusion-weighted sequences (white arrows). (C) Admission blood film showing fragmented red blood cells (black arrows). (D) Hematoxylin and eosin stain of renal biopsy from patient shows pathological microvascular changes with endothelial swelling, luminal narrowing, and trapped red blood cells (black arrow; bar represents 25 μm). (E) Patients who developed TMA, including patients from an independent cohort,7 received a higher weight-adjusted dose than unaffected multiple sclerosis patients treated with the same IFN-β preparation. *P < .001, Student t test. (F) All UK reports of TMA associated with IFN-β are associated with IFN-β1a dose >50 μg/wk, and 92% are associated with the highest available dose (n = 15 reports). (G-H) Evidence of activation of IFN response in renal biopsy of affected patient (H: MxA immunohistochemistry, red; bar represents 10 μm), with biopsy from patient with TMA not associated with IFN (G: genetic atypical hemolytic uremic syndrome [HUS]) for comparison.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/128/24/10.1182_blood-2016-05-715987/4/m_blood715987f1.jpeg?Expires=1765965110&Signature=ops5-9C-GIpq5VTI3L6f~EYJHxOZOeU-DHVMU-Gucf72qzdaOjd388FVHDFYQHoBQC8ACoJWFQIybbbCgampr-nQH4i-ZCS10eYlDmrYj~eqOgbRuv4pt6QLTLZ9uz-v11-r6jhnrwO4J6P5m-mldmr434kTRxQBeqsb91foC0oTxJHSgLDndSCec4DYpAvpypwtMeU4nJkZUvLGMsXvuMGDzK7kZ6L2qZ9GE-GcMCfO4HrfLprHs3O4GKzmzPa5mDNZ-Zs1rn4IdZ1xnU40SfjY1QIJ3hPnilw~kV2-ztbGxv1vt2SlpS-wIUI5EDRhrIX-37HHfrYXvTQeBrfazQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 3. Type I IFN signaling is species specific, limiting interpretation of cross-species preclinical toxicity models. Type I IFN proteins (-α and -β) act through a common receptor (IFNAR), leading to activation of downstream IRGs. (A) Preclinical toxicity testing of human recombinant type I IFN utilizes cross-species testing. Human recombinant type I IFN does not elicit a downstream IFN response in mouse endothelial cells, as measured by quantitative PCR for type I IRGs in BEND.5 brain endothelial cell line. Despite extensive preclinical testing (355 mice tested with human IFN-α, 347 mice tested with human IFN-β), no reports of small vessel toxicity were observed in publically available preclinical tests for human recombinant type I IFN therapies tested in rodents.31,32 (B) In contrast, dose-dependent upregulation of IRGs is observed in mouse endothelial cells exposed to same-species recombinant type I IFN in vitro (data represent mean ± standard error of the mean [SEM]; *P < .05, Student t test compared with no IFN; n = 3 experiments, IFN-α shown).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/128/24/10.1182_blood-2016-05-715987/4/m_blood715987f3.jpeg?Expires=1765965110&Signature=mNY683tStyl7T-VB04GlIN5HD37Bynvgp5mueSaJjH7YzOFWSXLzY8qfz3VdxDE1hfhvlkOjaMoa1VFf5yGN3nf5w7biToYTGEtXA1q~8SxiC-CG-U9BXz-OGYeDULniS402t83ONzR7M7ex0xDCrs51qtekAM8q1CN9BYN79vxC9jsxLfXMbYpjZaJtj4uWBYHUt660hu-CrFn-fbv5FiaAsofhek8whNs5nFPZ0V3SydkZzoxZMbHXDJMEYPllCz4z19CSZloyuvF5udCycvbCntyY-1imOzlTs8bA~Cqzxn9hgK8gT32pAuTeNo3fsNczgbVCxtmdUv-UHjNPyg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)