Although drug-induced thrombotic microangiopathy (TMA) is commonly reported, evidence for a causal association is uncommonly documented. In this issue of Blood, Kavanagh et al use multiple methods to clearly establish a causal association of type I interferon with TMA.1
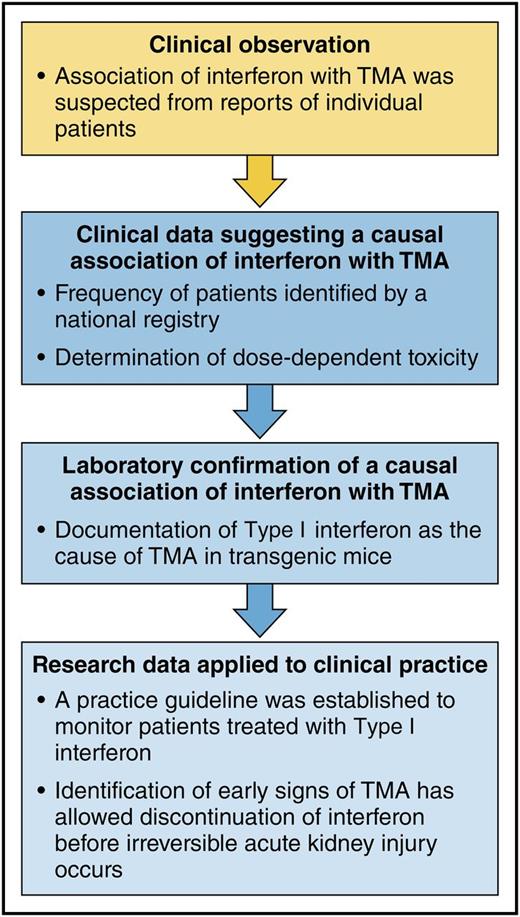
Establishing the evidence for type I interferon as a cause of TMA and using the results for improved patient care: a model for reciprocal translational research. The sequence of clinical and research investigations in the report by Kavanagh et al demonstrate the confirmation of clinical observations by a laboratory animal model and then further demonstrate the application of the research observations to clinical practice. This illustrates effective reciprocal translation, from bedside to bench and back again to the bedside. Professional illustration by Patrick Lane, ScEYEnce Studios.
Establishing the evidence for type I interferon as a cause of TMA and using the results for improved patient care: a model for reciprocal translational research. The sequence of clinical and research investigations in the report by Kavanagh et al demonstrate the confirmation of clinical observations by a laboratory animal model and then further demonstrate the application of the research observations to clinical practice. This illustrates effective reciprocal translation, from bedside to bench and back again to the bedside. Professional illustration by Patrick Lane, ScEYEnce Studios.
In the discipline of epidemiology, “cause” is a holy word, to be used only when definitive criteria for a causal association are met.2 The report by Kavanagh et al is remarkable for the multiple methods of their investigation, their definitive documentation of a causal association, and the importance of their findings for improved patient care.
As in many clinical investigations, their story began with patient observations. Among patients treated with type I interferon for multiple sclerosis, they noticed an unexpectedly high frequency of TMA associated with severe hypertension. The clinical features of TMA (microangiopathic hemolytic anemia, thrombocytopenia, and kidney injury) often presented after years of well-tolerated interferon treatment. In some patients, anemia, hypertension, and abnormal kidney function had been recognized for months before the onset of fulminant TMA.3 In spite of the onset of TMA years after its initiation in some patients, a causal association with interferon was suspected, because no other etiologies for TMA were apparent and no other drugs had been taken. Also, patients who developed TMA had received higher weight-adjusted doses of interferon. To support this clinical evidence, a transgenic mouse model for production of type I interferon demonstrated dose-dependent microvascular abnormalities comparable to TMA. A final step was to demonstrate that microvascular abnormalities in the transgenic mice required the presence of the type I interferon receptor. The causal association of type I interferon with TMA was established.
This knowledge was then applied to patient care. A program to monitor blood pressure and kidney function in multiple sclerosis patients treated with type I interferon was established in Scotland. This has allowed early recognition of abnormalities associated with interferon-induced TMA. Interferon treatment was stopped at the first sign of toxicity, when organ damage may be reversible. Since implementation of this program, no patients have developed fulminant organ failure due to interferon-induced TMA.
This report is a model for reciprocal translational research. It began with patient observations that supported a hypothesis for the causal association of interferon with TMA. Next, both clinical and laboratory research data documented the causal association. Finally, this knowledge was applied to develop a clinical practice guideline for early detection of interferon toxicity, hopefully preventing the occurrence of irreversible kidney injury. This reciprocal translational model can be described as bedside to bench and then back again to the bedside (see figure).
Beyond the story of interferon-induced TMA, this report also has lessons for understanding the TMA syndromes. Although TMA syndromes are all defined by similar pathologic and clinical features, they have multiple, diverse etiologies. Familiar TMA syndromes include thrombotic thrombocytopenic purpura, Shiga toxin–induced hemolytic uremic syndrome, and complement-mediated TMA. Drugs can also cause TMA, either by acute, immune-mediated reactions or by dose-dependent toxicity.4 A causal association of a drug with immune-mediated TMA can be established by the occurrence of repeated acute episodes with repeated exposures to the drug and by documentation of drug-dependent antibodies. A causal association of a drug with dose-dependent, toxicity-mediated TMA is more difficult to document. Our recent systematic review of all reports of drug-induced TMA identified 387 articles describing 586 patients with TMA attributed to 78 drugs.5 For 56 (72%) of these drugs, data supporting a clear causal association were not reported. In many reports, it appeared that the existence of multiple previous reports was the principal reason for assuming a causal association of the drug with TMA. This, of course, does not provide evidence but only perpetuates the presumption of causal association.
Compelling evidence for a causal association has been reported for very few drugs. Among drugs reported to cause TMA with clinical characteristics of an immune-mediated etiology, only quinine has been documented to cause TMA by the occurrence of repeated episodes following repeated quinine exposure and by documentation of quinine-dependent antibodies reactive with multiple cells.5 Among drugs reported to cause TMA with clinical characteristics of a dose-dependent toxicity etiology, only bevacizumab has been documented to cause TMA using rigorous methodology6 comparable to the current report of Kavanagh et al. Before the report by Kavanagh et al, 29 articles had described 40 patients with TMA attributed to type I interferon; none presented the convincing evidence for a causal association that is described in this report.
The report by Kavanagh et al not only is important for documenting the causal association of interferon with TMA but also is an important role model for investigating and understanding the pathogenesis of drug-induced TMA.
Conflict-of-interest disclosure: The author declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal