In this issue of Blood, Chu et al show that oxygen, by changing the conformation of hemoglobin (Hb), modulates many red cell properties by altering its interaction with band 3, the major red cell membrane protein.1
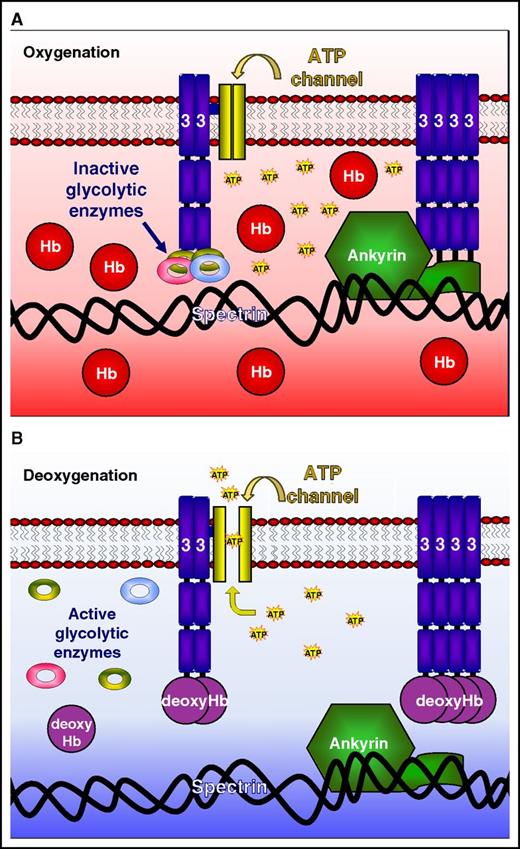
The molecular switch regulating oxygen-dependent red cell properties. (A) Oxygenated Hb has a lower affinity for band 3, so that glycolytic enzymes and ankyrin remain strongly bound and the ATP release channel is closed. (B) On deoxygenation, Hb has a higher affinity for band 3, displacing glycolytic enzymes and ankyrin and opening the ATP release channel. Glycolysis is stimulated, the cytoskeleton weakens, and ATP is released. The conformational change of Hb on transition from oxygenated to deoxygenated states, via reversible band 3 binding, therefore represents the molecular switch regulating red cell properties. Figure provided by Haiyan Chu and Philip S. Low.
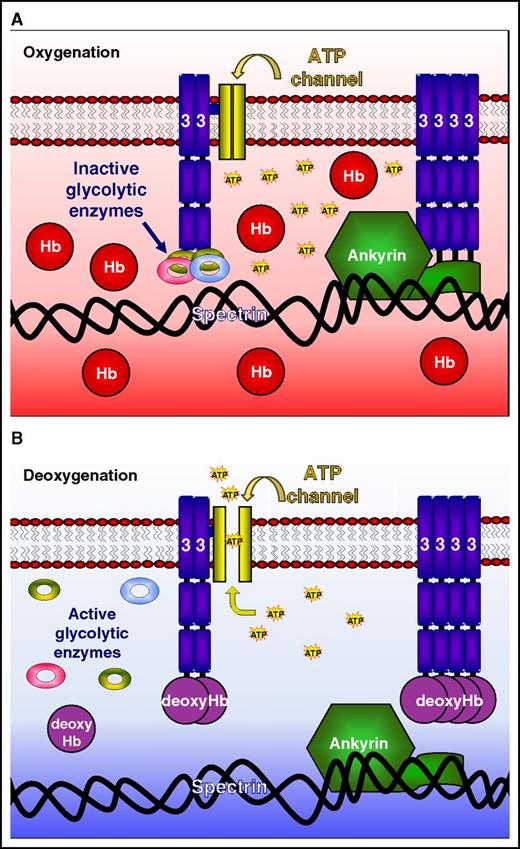
The molecular switch regulating oxygen-dependent red cell properties. (A) Oxygenated Hb has a lower affinity for band 3, so that glycolytic enzymes and ankyrin remain strongly bound and the ATP release channel is closed. (B) On deoxygenation, Hb has a higher affinity for band 3, displacing glycolytic enzymes and ankyrin and opening the ATP release channel. Glycolysis is stimulated, the cytoskeleton weakens, and ATP is released. The conformational change of Hb on transition from oxygenated to deoxygenated states, via reversible band 3 binding, therefore represents the molecular switch regulating red cell properties. Figure provided by Haiyan Chu and Philip S. Low.
Several roles of oxygen are widely appreciated. Oxygen’s participation in tissue metabolism via oxidative phosphorylation is well known, as is its carriage by red cell Hb. Oxygen may also take part in the generation of reactive oxygen species, the threat of which is counteracted by various antioxidant systems, of which necessarily there are several inside red cells. These oxidants may also have important physiological functions, especially in cell signaling. A third essential role of oxygen, however, has so far eluded most standard accounts of respiration—namely its regulatory function in many other aspects of red cell physiology, central to its multiple roles in the circulation. In effect, oxygen constitutes a “molecular switch” to modulate red cell properties. This third role of oxygen is addressed by the paper of Chu et al in the current issue of Blood. These authors present a major advance in the elucidation of the mechanism underlying this important role of oxygen (see figure).
The respiratory function of red cells involves 3 key proteins: Hb, carbonic anhydrase, and the anion exchanger (also known as band 3). Hemoglobin, of course, with its reversible oxygen binding and Bohr and Haldane effects, is central to the transport of both blood gases. Of the several billion molecules of Hb in each red cell, bulk cytoplasmic Hb occupying around a third of red cell volume participates in this role. As we see in the present paper of Chu et al, the much smaller amounts of membrane-associated Hb may, however, have a fundamentally different function.
Band 3, the anion exchanger, represents the most abundant protein in the red cell membrane, with a copy number of a few million. It exchanges Cl− for HCO3−, and therefore, participates in the carriage of blood gases. However, in addition, band 3 functions as a structural scaffold interacting with a variety of other proteins, including glycolytic enzymes, the spectrin-actin cytoskeleton via ankyrin, and also Hb.2-5 The N-terminal tail of band 3 contains many acidic amino acid residues, which are critical for these associations.2,6 Change in oxygen tension over physiological values modifies these interactions, as well as other red cell functions, notably solute permeability.7,8
The tetrameric shape of Hb alters with oxygen tension, so that on deoxygenation a central cavity opens between the 2 α/β-dimers. 2,3-Biphosphoglycerate binds at this site, reducing Hb’s oxygen affinity. This cavity also interacts with the negatively charged N-terminus of band 3. As for 2,3-biphosphoglycerate, deoxygenated Hb has a higher affinity for this N-terminus than when it is oxygenated. Thus, deoxygenated Hb has been shown in vitro to reduce the association of band 3 with some of its target proteins, including glycolytic enzymes and ankyrin. This association therefore represents an obvious mechanism for how changes in oxygen tension may modulate a number of key red cell functions.7 Deoxygenated Hb has previously been shown to switch glucose metabolism from glycolysis to the pentose phosphate shunt3,9 and also to reduce the interaction with ankyrin so weakening the cytoskeleton.5 Until now, however, testing the veracity of this hypothesis in vivo has not been possible.
Low’s group has long been interested in oxygen and red cell function. Their numerous important contributions to the field include work on the oxygen dependence of glucose metabolism and cytoskeletal integrity, in addition to much work on the structure of band 3.2-6 Here, Chu et al make use of transgenic mice expressing the N-terminus of human band 3 either containing or lacking the binding site for deoxygenated Hb to address its role in vivo. In a series of elegant experiments, they show that the presence of the band 3 binding site for human Hb is required for mouse red cells to react to changes in oxygen tension with regard to 3 important red cell properties: glycolysis, integrity of the cytoskeleton, and adenosine triphosphate (ATP) release. They thus provide compelling evidence that reversible binding of Hb to band 3, modulated through its oxygen-dependent shape change, constitutes the molecular switch that controls these aspects of red cell behavior.
As always, further questions arise. As Chu et al comment, the important effects of oxygen on red cell solute permeability in both normal and diseased red cells, notably those from patients with sickle cell disease, await definition. In addition, it remains to be determined how animals lacking both band 3 and tetrameric Hb, like lampreys,10 are nevertheless able to respond to oxygen at least with changes in solute permeability. Finally, the importance of this Hb/band 3 interaction for the development and longevity of the red cell, and its various roles in the circulation, awaits elucidation. Notwithstanding, the current work represents a major advance in defining Hb/band 3 interactions as an important molecular switch in regulation of red cell properties.
Conflict-of-interest disclosure: The author declares no competing financial interests.