Abstract
Introduction
Achieving MRD-negative remission after FCR therapy is an important predictor of longer progression-free (PFS) and overall survival (OS); however, with long-term follow-up, many MRD-negative patients subsequently relapse. The time course and predictive factors for relapse in initially MRD-negative patients have not been extensively characterized.
Methods
We prospectively analyzed 289 patients treated first-line with FCR for CLL at M.D. Anderson Cancer Center from 2008-15, to determine pre-treatment characteristics associated with post-treatment MRD negativity and the time course of relapse in MRD-negative patients. MRD analysis was performed in BM after course 3 (n=239) and at end of therapy (EOT) (n=231) using standardized 4-color flow cytometry (FLC). Ninety-five MRD-negative patients had 12-monthly serial MRD monitoring on blood.
Results
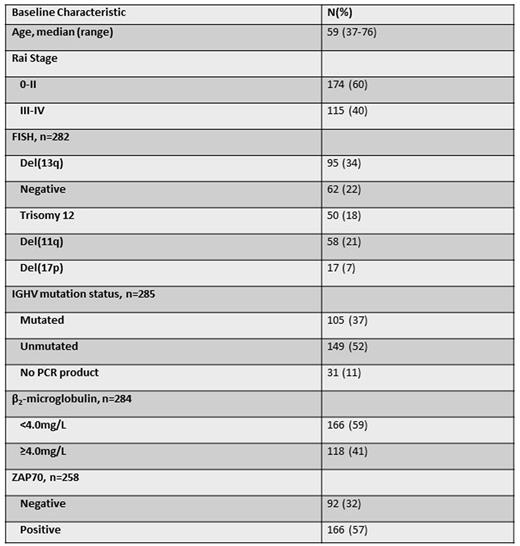
Pretreatment characteristics are shown below (N=289).
For the total cohort, the only pre-treatment characteristic significantly associated with PFS was IGHV-unmutated (IGHV-UM) (HR 3.0 [1.6-5.5], p<0.001). Analyzed separately, among IGHV-mutated (IGHV-M) patients, only ZAP70 positivity (38/92 [41%]) by immunohistochemistry was significantly associated with shorter PFS (HR 3.2 [1.3-8.2], p=0.01); among IGHV-UM patients only B2M ≥4.0mg/L (64/146 [44%]) was significantly associated with shorter PFS (HR 1.9 [1.2-3.0], p=0.005).
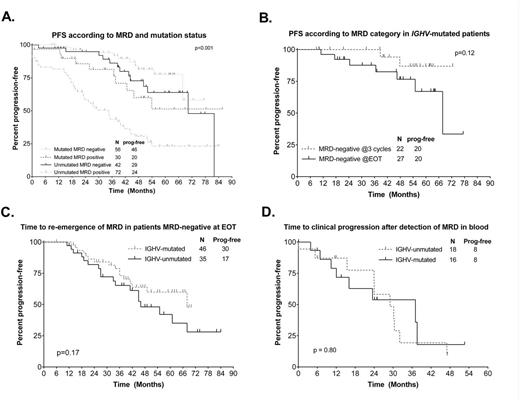
Overall response rate was 96%. 46/239 patients (19%) after 3 courses and 120/231 (51%) at EOT achieved MRD negativity in BM. The following pre-treatment characteristics were significantly associated in univariableanalysis with achieving MRD-negativity at EOT in all patients: negative FISH (OR 3.1 [1.3-7.0], p=0.01), trisomy 12 (OR 2.7 [1.1-6.3], p=0.03, IGHV-M [OR 3.2 (1.8-5.9), p<0.001], β2-microglobulin <4.0mg/L [OR 1.85 (1.1-3.1), p=0.02] and ZAP70 negativity [OR 1.8 (1.03-3.2), p=0.04]. On multivariable analysis, only IGHV-M was significantly associated with MRD-negativity [OR 3.7 (1.7-8.2), p=0.001]. There were trends toward association between negative FISH [OR 5.4 (0.9-31.8), p=0.07] and trisomy 12 [OR 4.9 (0.8-29.8), p=0.08] with MRD-negativity. MRD negativity at EOT correlated with PFS, independent of IWCLL response category, and correlated with PFS in both IGHV-M and IGHV-UM patients (Figure A). In IGHV-M patients who were MRD-negative at EOT, there was a trend toward superior PFS if MRD-negativity was attained after 3 courses of FCR, even though 11/22 patients who were MRD-negative after 3 courses received no further therapy and only 3/22 received 6 courses (Figure B).
Re-emergence of MRD in blood was detected in 45/95 MRD negative patients, at a median of 49 months post- treatment; this appeared less frequent in IGHV-M patients (Figure C). Re-emergence of MRD preceded clinical relapse by a median of 25 months. There were no significant differences in time-to-subsequent clinical relapse after re-emergence of MRD according to mutation status (Figure D). Among IGHV-M patients who were MRD-negative after 3 cycles, 4/22 had subsequent re-emergence of MRD.
Conclusions
Among patients with CLL receiving first-line FCR, the best pre-treatment predictor of achieving MRD-negativity and subsequent longer PFS was IGHV-M. Post-treatment MRD-negativity correlated with subsequent PFS; patients with IGHV-M who achieved MRD-negativity after 3 courses had a particularly favorable outcome, even after abbreviation of therapy. However, despite achieving MRD-negativity, many patients subsequently relapse; serial MRD monitoring in peripheral blood can herald relapse with a lead-time of approximately 2 years and potentially direct monitoring and/or early intervention strategies.
Thompson:Pharmacyclics: Consultancy, Honoraria. O'Brien:Janssen: Consultancy, Honoraria; Pharmacyclics, LLC, an AbbVie Company: Consultancy, Honoraria, Research Funding. Jain:Incyte: Research Funding; Celgene: Research Funding; Seattle Genetics: Research Funding; Servier: Consultancy, Honoraria; Abbvie: Research Funding; Pharmacyclics: Consultancy, Honoraria, Research Funding; Infinity: Research Funding; Novimmune: Consultancy, Honoraria; BMS: Research Funding; Pfizer: Consultancy, Honoraria, Research Funding; Novartis: Consultancy, Honoraria; Genentech: Research Funding; ADC Therapeutics: Consultancy, Honoraria, Research Funding. Kadia:Novartis: Honoraria; BMS: Research Funding. DiNardo:Novartis: Research Funding; Daiichi Sankyo: Research Funding; Agios: Research Funding; Abbvie: Research Funding; Celgene: Research Funding. Wierda:GSK/Novartis: Consultancy, Research Funding; Abbvie: Consultancy, Research Funding; Gilead: Consultancy, Research Funding; Genentech/Roche: Consultancy, Research Funding; Pharmacyclics: Consultancy, Research Funding; Emergent: Consultancy, Research Funding; Sanofi: Consultancy; Celgene: Consultancy; Genzyme: Consultancy; Merck: Consultancy; Karyopharm: Research Funding; Acerta: Research Funding; Janssen: Research Funding; Juno Therapeutics: Research Funding; KITE Pharma: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.