Abstract
Introduction: Therapies able to improve overall survival in patients with relapsed/refractory (RR) CLL are needed. We have previously reported that idelalisib (IDELA), a selective PI3K delta inhibitor, administered in combination withbendamustine/rituximab (BR) improves progression-free survival compared with BR alone after a medianfollowup of 12 months. This study (NCT01569295) was unblinded by the independent data monitoring committee at first interim analysis for efficacy. We now present updated data on overall survival (OS).
Methods: Between June 2012 and August 2014, 416 patients (pts) with RR CLL were enrolled in the study across 19 countries. The current analysis data cutoff date of May 2016 represents a median follow-up of 21 months. Progression-free survival based on independent review committee assessment was the primary endpoint of this study, with OS as a secondary endpoint. All pts had completed study treatment with BR. Key eligibility criteria included pts with RR CLL requiring therapy, having received previous purine analog or bendamustine (ineligible if refractory to bendamustine); and anti-CD20 antibody; relapsing or progressing within 36 months of the completion of the last therapy. Patients were randomized to BR for 6 cycles Q 28 days (B = 70 mg/m2 D1, D2 of each cycle; R = 375 mg/m2 C1 and 500 mg/m2 C2-6) and IDELA 150 mg BID or placebo (administered until IRC-confirmed PD), death, intolerable toxicity, or withdrawal of consent. Stratification was based on presence/absence ofdel(17p) and/or p53 mutation (mut), immunoglobulin heavy chain variable region (IGHV) mutated/unmutated (analysis performed by a central lab), and disease status refractory (CLL progression <6 months from completion of prior therapy) vs relapsed (CLL progression ≥6 months from completion of prior therapy). Crossover was not permitted at the time of PD or unblinding.
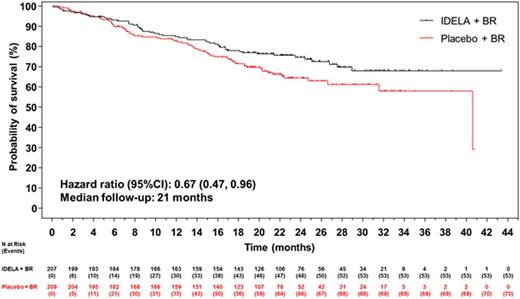
Results: The ITT population reflects 207/209 pts in the IDELA + BR/BR + placebo arm: 76% male; 42% ≥65 years; Rai stage III/IV 46%; median time since completion of last prior therapy 16 months; pts with high-risk features (del[17p]/p53mut 32.9%, unmutated IGHV 83.2%, refractory 29.8%); median number of prior therapies: 2 (range 1-13); and median follow-up 21 months. All pts have completed study treatment with BR. A total of 65 pts remain on study treatment: 64 on IDELA monotherapy and 1 pt on placebo. Overall by ITT and IRC, 260/416 pts (IDELA/placebo 95/165) have met the primary endpoint of PD or death. Median OS (mo) of IDELA + BR vs BR + placebo was not reached vs 41 (HR = 0.67; p value 0.036; 95% CI 0.47, 0.96) (Figure 1). The safety findings were similar to what we previously reported: Serious AEs occurred in 147 (71%)/94 (5%) IDELA/placebo arms, respectively. The commonly occurring SAEs by system organ class were infections and infestations (41%/23%) and by MEDRA-preferred terms febrile neutropenia 43 (21%)/10 (5%) and pneumonia 35 (17%)/16 (8%) in the IDELA/placebo arms respectively. The total number of pts with opportunistic infections (Pneumocystis jirovecii pneumonia [PJP]/cytomegalovirus [CMV]) in the IDELA arm was 5/13 vs 0/3 in the placebo arm.
Conclusion: IDELA in combination with BR is superior to BR alone with regard to OS in RR CLL. The improvement in OS was observed across risk categories. Opportunistic infections (PJP, CMV) and SAEs were more frequent in the IDELA vs placebo arm. Results of IDELA-containing regimens may be further improved with implementation of adequate PJP prophylaxis and CMV monitoring measures. This regimen represents an important new option for pts with RR CLL.
Zelenetz:Gilead Sciences: Research Funding. Brown:Celgene: Consultancy; Sun BioPharma: Consultancy; Pfizer: Consultancy; Roche/Genentech: Consultancy; Gilead Sciences: Consultancy; Janssen: Consultancy; Infinity: Consultancy; Abbvie: Consultancy. Delgado:Roche: Consultancy, Honoraria, Research Funding; Janssen: Consultancy, Honoraria; Gilead Sciences: Consultancy, Honoraria; GSK/Novartis: Honoraria; Abbvie: Consultancy. Eradat:Genentech: Consultancy, Honoraria, Speakers Bureau; AbbVie: Consultancy, Honoraria, Research Funding, Speakers Bureau; Gilead: Consultancy, Honoraria, Research Funding, Speakers Bureau; Pharmacyclics: Consultancy, Honoraria. Ghia:Adaptive: Consultancy; Gilead: Consultancy, Honoraria, Research Funding, Speakers Bureau; Janssen: Consultancy, Honoraria, Speakers Bureau; Roche: Honoraria, Research Funding; Abbvie: Consultancy, Honoraria. Jurczak:Celltrion, Inc: Research Funding; Acerta: Research Funding; Gilead Sciences: Research Funding; Bayer: Research Funding; Janssen: Research Funding. Loscertales:Roche: Honoraria, Speakers Bureau; Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Gilead Sciences: Honoraria, Membership on an entity's Board of Directors or advisory committees. MacDonald:Gilead Sciences: Speakers Bureau. Morschhauser:Celgene: Consultancy, Honoraria; Gilead Sciences: Consultancy, Honoraria; Servier: Consultancy, Honoraria; Roche: Consultancy, Honoraria; Janssen: Honoraria. De la Serna:Abbvie: Consultancy; Roche: Consultancy, Speakers Bureau; Janssen: Consultancy, Speakers Bureau; Gilead Sciences: Consultancy, Speakers Bureau. Shadman:Pharmacyclics: Honoraria, Research Funding. Pocock:Gilead Sciences: Other: Sponsorship to attend the EHA 2016 Meeting; Janssen: Speakers Bureau; Takeda: Honoraria. Adewoye:Gilead Sciences: Employment, Equity Ownership. Kim:Gilead Sciences: Employment, Equity Ownership. Simpson:Celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees; Roche: Membership on an entity's Board of Directors or advisory committees. Stilgenbauer:Boehringer Ingelheim: Consultancy, Honoraria, Other: Travel grants , Research Funding; Pharmacyclics: Consultancy, Honoraria, Other: Travel grants , Research Funding; Novartis: Consultancy, Honoraria, Other: Travel grants , Research Funding; AbbVie: Consultancy, Honoraria, Other: Travel grants, Research Funding; GSK: Consultancy, Honoraria, Other: Travel grants , Research Funding; Mundipharma: Consultancy, Honoraria, Other: Travel grants , Research Funding; Hoffmann-La Roche: Consultancy, Honoraria, Other: Travel grants , Research Funding; Sanofi: Consultancy, Honoraria, Other: Travel grants , Research Funding; Gilead: Consultancy, Honoraria, Other: Travel grants , Research Funding; Janssen: Consultancy, Honoraria, Other: Travel grants , Research Funding; Celgene: Consultancy, Honoraria, Other: Travel grants , Research Funding; Genzyme: Consultancy, Honoraria, Other: Travel grants , Research Funding; Genentech: Consultancy, Honoraria, Other: Travel grants , Research Funding; Amgen: Consultancy, Honoraria, Other: Travel grants, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal