Key Points
Thrombin and APC elicit paradoxical signaling responses through cleavage of PAR1 at different sites.
EPCR occupancy mediates β-arrestin-2 biased protective PAR1 signaling by both proteases via recruiting GRK5 and Dvl-2.
Abstract
Activation of protease-activated receptor 1 (PAR1) by activated protein C (APC) and thrombin elicits paradoxical cytoprotective and cytotoxic signaling responses in vascular endothelial cells through cleavage of the receptor at Arg-46 and Arg-41 protease recognition sites, respectively. It has been reported that unlike a disruptive G-protein-mediated PAR1 signaling by thrombin, APC induces a protective β-arrestin-2 biased PAR1 signaling by unknown mechanisms. We hypothesize that the occupancy of endothelial protein C receptor (EPCR) by the Gla-domain of protein C/APC is responsible for the β-arrestin-2 biased PAR1 signaling independent of the protease cleavage site. To test this hypothesis, we monitored the signaling specificity of thrombin in endothelial cells in response to lipopolysaccharide (LPS) with or without pretreatment of cells with protein C-S195A. The PAR1-dependent recruitment of β-arrestin-2 in response to LPS by both APC and thrombin was analyzed by functional, gene silencing, and signaling assays. Results indicate that similar to APC, thrombin exerts cytoprotective effects via β-arrestin-2 biased PAR1 signaling. Similar to APC, thrombin triggered β-arrestin-2-dependent recruitment of disheveled 2 (Dvl-2) in PC-S195A pretreated cells. Further studies in HeLa cells transfected with PAR1 constructs revealed that EPCR occupancy initiates β-arrestin-2 biased PAR1 signaling independent of the protease cleavage sites. We demonstrate that EPCR occupancy recruits G-protein coupled receptor kinase 5, thereby inducing β-arrestin-2 biased PAR1 signaling by both APC and thrombin. In support of a physiological relevance for these results, intraperitoneal administration of PC-S195A conferred a cytoprotective effect for thrombin in an in vivo inflammatory model.
Introduction
When the procoagulant protease thrombin binds to endothelial cell surface receptor, thrombomodulin (TM), the resulting protease-cofactor complex no longer functions in the procoagulant pathway, but instead it initiates the anticoagulant pathway by activating protein C in complex with endothelial protein C receptor (EPCR) to activated protein C (APC).1-6 The interaction of TM with thrombin not only changes the procoagulant substrate specificity of the protease but also alters its proinflammatory substrate specificity by occupying exosite-1 of thrombin, which is required for thrombin interaction and activation of protease-activated receptor 1 (PAR1).7-10 Interestingly, APC in complex with EPCR can also activate PAR1.10-12 In this case, however, cleavage of PAR1 by APC-EPCR complex elicits anti-inflammatory responses in endothelial cells.10-12 The mechanism through which activation of the same G-protein coupled receptor (GPCR), by thrombin and APC elicits paradoxical signaling responses is the subject of intensive investigation by several groups. This question is confounded by the observation that thrombin activates PAR1 faster than APC by at least 3 orders of magnitude.13,14 A partial answer for this question was provided by findings that receptors of protein C activation and APC signaling pathways have all been localized in lipid rafts of endothelial cells and that activation of protein C–EPCR complex by thrombin-TM complex mechanistically links activation of the anticoagulant protein to its anti-inflammatory signaling.15-17 However, a key insight into the mechanism of differential PAR1 signaling by the 2 proteases has recently come from a study showing that PAR1-dependent cytoprotective activity of APC may not be mediated through either one of G-proteins but rather via β-arrestin-2 biased signaling.18 The mechanism by which APC, but not thrombin, initiates β-arrestin-2 biased PAR1 signaling is not known. Recent results indicated APC cleavage of PAR1 at the noncanonical Arg-46 cleavage site, rather than the canonical Arg-41 site, may be responsible for the cytoprotective β-arrestin-2 biased signaling by APC, but not thrombin.19,20
In this study we hypothesize that occupancy of EPCR by the Gla-domain of protein C is responsible for a GPCR kinase (GRK)–dependent recruitment of β-arrestin-2 to the cytoplasmic domain of PAR1 upon its cleavage by APC and that thrombin will also signal through β-arrestin-2 biased signaling if EPCR is occupied by protein C. To test this hypothesis, we pretreated endothelial cells with the catalytically inactive protein C-S195A (PC-S195A) zymogen before analyzing signaling specificity of PAR1 in response to thrombin. Consistent with the hypothesis, we demonstrate that occupancy of EPCR by either APC or PC-S195A results in activation of GRK5, thereby recruiting β-arrestin-2 and disheveled 2 (Dvl-2) scaffolding proteins and initiating biased PAR1 signaling by both APC and thrombin independent of the proteases cleaving at either Arg-41 or Arg-46 sites. We demonstrate that EPCR-dependent cytoprotective activity of thrombin through PAR1 may have physiological significance because intraperitoneal administration of PC-S195A endows a barrier-protective effect for thrombin in an in vivo model.
Materials and methods
Expression of PAR1 mutants
Permeability assay
EA.hy926 cell culture and permeability in response to lipopolysaccharide (LPS) was assessed by spectrophotometric measurement of the flux of 0.5 mL Evans blue-bound albumin (0.67 mg/mL diluted in growth medium containing 4% bovine serum albumin) across functional cell monolayer by a modified 2-compartment chamber model as described.21
siRNA and shRNA silencing
Short interfering (siRNA) and short hairpin RNA (shRNA) knockdowns of β-arrestin-1 and 2, sphingosine 1-phosphate receptor (S1P1), Dvl-2, PAR1, GRK5, and GRK6 in endothelial cells were performed as described.22
Cytosolic and membrane protein extraction
The cytoplasmic and membrane proteins were prepared by a modified subcellular fractionation protocol as described.23
Clonogenic assay for cell survival and cytoprotective function
Clonogenic assays were performed for assessing cell survival in control and siRNA-silenced endothelial cells as described.24
In vivo permeability assay
Eight- to 10-week-old male C57bl/6 mice were used for in vivo studies after a 5-day acclimatization period as described.22
Supplemental materials
List of reagents and detailed description of signaling assays are described as supplemental Materials (available on the Blood Web site).
Results
Occupancy of EPCR by protein C alters specificity of PAR1 signaling
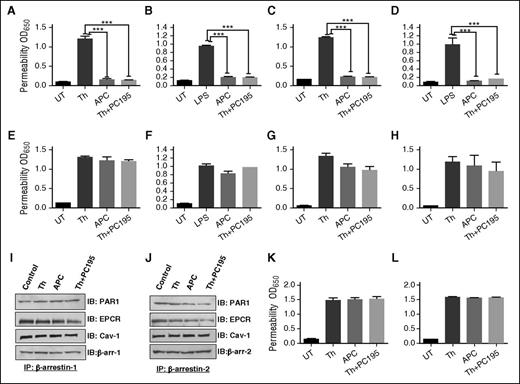
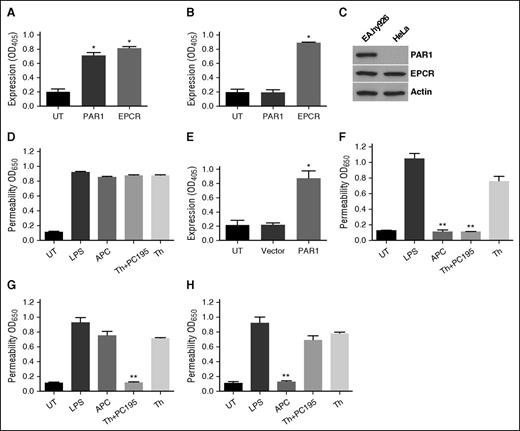
APC exerts its PAR1-dependent cytoprotective effect in endothelial cells through a β-arrestin-2 biased signaling mechanism.18-20 By contrast thrombin couples PAR1 cleavage to a G-protein-dependent signaling event.25,26 We hypothesize that occupancy of EPCR by its ligand protein C/APC is responsible for β-arrestin-2 biased PAR1 signaling and that thrombin would also signal by the same mechanism if EPCR is occupied. To test this hypothesis, we pretreated β-arrestin-1- and β-arrestin-2-silenced cells with PC-S195A before monitoring PAR1-dependent signaling by thrombin in cell permeability assay. In agreement with previous results,21 pretreatment of endothelial cells with PC-S195A switched signaling specificity of thrombin; thus, similar to APC, thrombin elicited a cytoprotective effect in cells in which hyperpermeability was induced with either high concentrations of thrombin (Figure 1A) or LPS (Figure 1B). Cytoprotective activity of thrombin + PC-S195A, similar to APC, was mediated through β-arrestin-2 biased PAR1 signaling because silencing β-arrestin-1 had no effect (Figure 1C-D); however, β-arrestin-2 silencing abrogated cytoprotective activity of both APC and thrombin + PC-S195A (Figure 1E-F). Cytoprotective activity of thrombin + PC-S195A, similar to APC, was PAR1- and EPCR-dependent because function-blocking antibodies to either receptor abrogated protective effects (Figure 1G-H). Thrombin is known to elicit a PAR1-dependent protective activity at a low concentration of ∼50 pM.27 Interestingly, siRNA silencing studies indicated that this activity of thrombin is not mediated through β-arrestin-2 biased signaling (data not presented).
EPCR occupancy regulates β-arrestin-2 biased PAR1 signaling by APC and thrombin. Confluent EA.hy926 endothelial cells were transfected with the control siRNA (A-B) or siRNA specific for either β-arrestin-1 (C-D) or β-arrestin-2 (E-F) before treating cells with the PAR1 agonists (2 nM thrombin, 20 nM APC, or 2 nM thrombin + 50 nM PC-S195A for 3 hours) and monitoring cell permeability in response to either thrombin (10 nM for 10 minutes in panels A, C, E) or LPS (10 ng/mL for 4 hours in panels B, D, F) as described in “Materials and methods.” (G-H) The same as previous panels except that cell permeability in response to thrombin (10 nM) was carried in the presence of function-blocking anti-EPCR or anti-PAR1 antibodies. (I-J) sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE), immunoprecipitation, and immunoblotting of lysates derived from cells treated with different agonists. Total cellular proteins, derived from nontreated cells or cells treated with thrombin (2 nM), APC (20 nM), and thrombin (2 nM) + PC-S195A (50 nM), were immunoprecipitated with anti-β-arrestin-1 (I) or anti-β-arrestin-2 (J) and separated on SDS-PAGE followed by immunoblotting with anti-PAR1, anti-EPCR, anti-caveolin-1, or different pairs of the same anti-β-arrestin antibodies. (K) The same as panel A, except that permeability in response to thrombin (10 nM) was monitored in endothelial cells treated with MβCD (10 mM for 1 hour) before incubation with the agonists. (L) The same as previous panel, except that permeability in response to thrombin (10 nM) was monitored in endothelial cells transfected with specific siRNA for S1P1 before incubation with the agonists. All results are shown as means ± standard deviation of 3 different experiments. ***P < .001. IB, immunoblotting; IP, immunoprecipitation; MβCD, methyl-β-cyclodextrin; PC195, PC-S195A; Th, thrombin; UT, untreated control.
EPCR occupancy regulates β-arrestin-2 biased PAR1 signaling by APC and thrombin. Confluent EA.hy926 endothelial cells were transfected with the control siRNA (A-B) or siRNA specific for either β-arrestin-1 (C-D) or β-arrestin-2 (E-F) before treating cells with the PAR1 agonists (2 nM thrombin, 20 nM APC, or 2 nM thrombin + 50 nM PC-S195A for 3 hours) and monitoring cell permeability in response to either thrombin (10 nM for 10 minutes in panels A, C, E) or LPS (10 ng/mL for 4 hours in panels B, D, F) as described in “Materials and methods.” (G-H) The same as previous panels except that cell permeability in response to thrombin (10 nM) was carried in the presence of function-blocking anti-EPCR or anti-PAR1 antibodies. (I-J) sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE), immunoprecipitation, and immunoblotting of lysates derived from cells treated with different agonists. Total cellular proteins, derived from nontreated cells or cells treated with thrombin (2 nM), APC (20 nM), and thrombin (2 nM) + PC-S195A (50 nM), were immunoprecipitated with anti-β-arrestin-1 (I) or anti-β-arrestin-2 (J) and separated on SDS-PAGE followed by immunoblotting with anti-PAR1, anti-EPCR, anti-caveolin-1, or different pairs of the same anti-β-arrestin antibodies. (K) The same as panel A, except that permeability in response to thrombin (10 nM) was monitored in endothelial cells treated with MβCD (10 mM for 1 hour) before incubation with the agonists. (L) The same as previous panel, except that permeability in response to thrombin (10 nM) was monitored in endothelial cells transfected with specific siRNA for S1P1 before incubation with the agonists. All results are shown as means ± standard deviation of 3 different experiments. ***P < .001. IB, immunoblotting; IP, immunoprecipitation; MβCD, methyl-β-cyclodextrin; PC195, PC-S195A; Th, thrombin; UT, untreated control.
Cytoprotective activity of proteases requires cross talk with Gi-coupled S1P1 in lipid rafts
We evaluated membrane association of β-arrestins with receptors by immunoprecipitation of total protein extracts, before and after treatment of cells with different ligands using anti-β-arrestin-1 and 2 antibodies followed by immunoblotting using antibodies to different receptors. Results indicated both β-arrestin-1 and 2 are associated with EPCR, PAR1, and caveolin-1 (Figure 1I-J). Cholesterol-depleting drug MβCD inhibited barrier-protective activity of both APC and thrombin + PC-S195A, supporting the hypothesis that PAR1- and EPCR-dependent cytoprotective signaling by both proteases requires receptor localization to lipid rafts (Figure 1K). These results are consistent with previous findings that receptors and signaling molecules involved in EPCR- and PAR1-dependent protective signaling are localized within lipid rafts.15,21 Furthermore, analysis of activity of APC and thrombin + PC-S195A in cell permeability assays in the presence of pertussis toxin suggested that protective activity of both proteases requires signaling cross talk through a GPCR that is coupled to Gi-protein (not shown). In agreement with previous results,28,29 we found the putative Gi-protein coupled GPCR is S1P1 because siRNA knockdown abrogated PAR1- and EPCR-dependent barrier-protective activity of both APC and thrombin (Figure 1L).
Dvl-2 is required for cytoprotective activity of APC and thrombin + PC-S195A
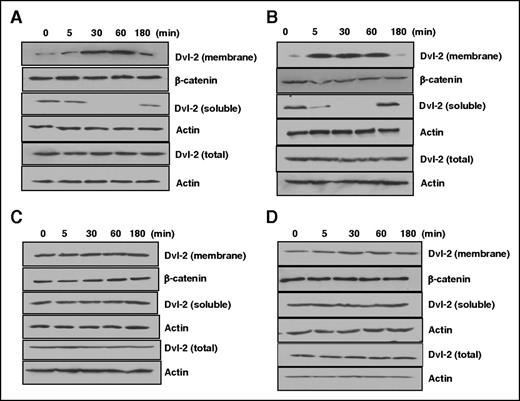
Dvl-2 expression and polymerization is required for APC-induced activation of Rac1, a small guanosine triphosphatase (GTPase) involved in cytoprotective function of APC.18,30 Immunofluorescence experiments have shown formation of Dvl-2 positive puncta in the cytoplasm upon APC treatment, which is accompanied by the loss of cytosolic Dvl-2 (soluble fraction) after 30 minutes.18 We found Dvl-2 silencing abrogates cytoprotective activity of both APC and thrombin + PC-S195A in hyperpermeability assays (induced by thrombin, Figure 1A; LPS, Figure 1B). To further investigate role of EPCR occupancy, cells were treated with APC or PC-S195A before stimulation with thrombin and analysis of cytoplasmic and membrane fractions of cell lysates by western blotting using anti-Dvl-2 antibody. Results suggested APC markedly decreases cytosolic level of Dvl-2 by a time-dependent manner (Figure 2A). Time-course analysis indicated Dvl-2 levels of membrane fractions is significantly increased, suggesting a redistribution of Dvl-2 to different subcellular locations upon exposure to APC. Accordingly, the amount of Dvl-2 in total cell lysates remained constant over the indicated time course, indicating the loss of Dvl-2 from the soluble fraction was not caused by Dvl-2 degradation. β-catenin and actin were used as loading controls for analysis of membrane and soluble fractions, respectively. A similar pattern of Dvl-2 redistribution was observed for thrombin in lysates of cells pretreated with PC-S195A. Thus, a similar level of decrease in the cytosolic Dvl-2 and increase in the membrane Dvl-2 was observed with thrombin + PC-S195A (Figure 2B). Although neither thrombin nor PC-S195A alone had any effect on Dvl-2 redistribution/subcellular localization (Figure 2C-D). Analysis of these results indicate thrombin + PC-S195A treatment accelerates subcellular redistribution of Dvl-2 much faster than APC because a significant decrease in the cytosolic fraction of Dvl-2 was observed within 5 minutes (Figure 2B), whereas a 30-minute APC treatment was required to observe a notable decrease in the Dvl-2 level (Figure 2A). Similarly, a faster accumulation of membrane Dvl-2 was detected in thrombin + PC-S195A-treated lysates within 5 minutes (Figure 2B), whereas almost 30 minutes lapsed before a buildup of Dvl-2 could be seen in APC-treated lysates (Figure 2A). Although neither control nor β-arrestin-1 siRNA inhibited thrombin + PC-S195A-mediated subcellular redistribution of Dvl-2, β-arrestin-2 siRNA abrogated this function (supplemental Figure 1A-C). Finally, silencing PAR1 also abrogated redistribution of thrombin-mediated Dvl-2 in cells pretreated with PC-S195A (supplemental Figure 1D), suggesting the PAR1 cleavage dependence of signaling by thrombin. These results indicate occupancy of EPCR confers PAR1-dependent cytoprotective function for thrombin through Dvl-2 and β-arrestin-2 biased signaling. Both APC and thrombin + PC-S195A also inhibited activation of NF-kB in LPS stimulated cells by β-arrestin-2 biased PAR1 signaling (not shown). All results presented previously for Figures 1 and 2 could be recapitulated if cells were treated with thrombin receptor agonist peptide (TRAP). Thus, TRAP alone elicited a disruptive response; however, TRAP induced a β-arrestin-2- and Dvl-2-dependent protective response if cells were pretreated with PC-S195A (not shown).
EPCR occupancy induces β-arrestin-2 dependent translocation of Dvl-2 to the plasma membrane independent of β-catenin. (A-D) Western blot analysis of cytoplasmic and membrane fractions of Dvl-2 in cell lysates using an anti-Dvl-2 antibody. EA.hy926 cells were treated with 20 nM APC (A), 2 nM thrombin + 50 nM PC-S195A (B), 2 nM thrombin alone (C), or 50 nM PC-S195A alone (D) for different times, and cell lysates were fractionated and analyzed by anti-Dvl-2 as described in “Materials and methods.” In all panels, the protein level of actin was monitored by an anti-actin antibody as a control for the total and cytosolic fraction of Dvl-2, whereas the β-catenin level was monitored as a control for the membrane fraction of Dvl-2.
EPCR occupancy induces β-arrestin-2 dependent translocation of Dvl-2 to the plasma membrane independent of β-catenin. (A-D) Western blot analysis of cytoplasmic and membrane fractions of Dvl-2 in cell lysates using an anti-Dvl-2 antibody. EA.hy926 cells were treated with 20 nM APC (A), 2 nM thrombin + 50 nM PC-S195A (B), 2 nM thrombin alone (C), or 50 nM PC-S195A alone (D) for different times, and cell lysates were fractionated and analyzed by anti-Dvl-2 as described in “Materials and methods.” In all panels, the protein level of actin was monitored by an anti-actin antibody as a control for the total and cytosolic fraction of Dvl-2, whereas the β-catenin level was monitored as a control for the membrane fraction of Dvl-2.
Analysis of cytoprotective activity of APC and thrombin + PC-S195A by adhesion and clonogenic assays
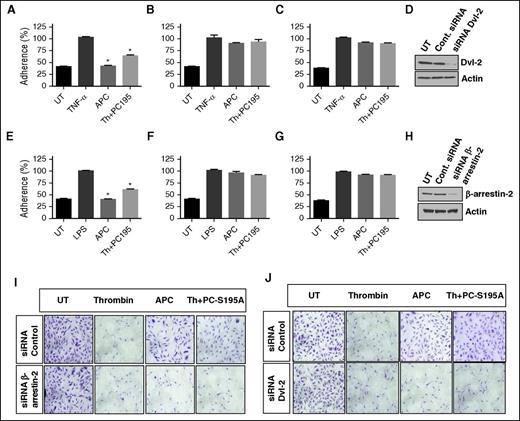
Silencing studies indicated that similar to APC, thrombin + PC-S195A inhibit adherence of monocytic THP-1 cells to tumor necrosis factor α (TNF-α)–stimulated endothelial cells by β-arrestin-2- and Dvl-2-dependent mechanisms (Figure 3A-H). These results are consistent with our published data that thrombin + PC-S195A inhibit TNF-α-stimulated expression of cell surface adhesion molecules VCAM-1, ICAM-1, and E-selectin in endothelial cells.31 Further studies showed that APC inhibits cytotoxic function of staurosporin by β-arrestin-2 biased signaling (Figure 3I, lower panel). In line with the hypothesis developed previously, β-arrestin-2-dependent cytoprotective function of both proteases is mediated through occupancy of EPCR because PC-S195A rendered thrombin protective by a similar mechanism (Figure 3I). In agreement with results presented previously, cytoprotective function of both APC and thrombin + PC-S195A in response to staurosporine required Dvl-2 because silencing Dvl-2 abrogated protective function (Figure 3J).
EPCR occupancy inhibits the adhesion of THP-1 cells to cytokine-activated EA.hy926 endothelial cells through Dvl-2 and β-arrestin-2 biased signaling. (A-C) TNF-α (10 ng/mL)–mediated adherence of THP-1 cells to EA.hy926 cell monolayers was analyzed after treating monolayers with APC (20 nM) or thrombin (2 nM) + PC-S195A (50 nM) after transfecting cells with control siRNA (A), β-arrestin-2 siRNA (B), or Dvl-2 siRNA (C). (D) Western blot analysis of the efficiency of silencing by the control siRNA or the siRNA specific for Dvl-2. (E-G) The same as panels A-C except that the adherence of THP-1 cells to LPS (10 ng/mL)–stimulated endothelial cells were monitored. (H) Western blot analysis of the efficiency of silencing by the control siRNA or the siRNA specific for β-arrestin-2. (I-J) Analysis of EPCR-dependent cytoprotective activity of APC and thrombin by the clonogenic assay. Following transfection of EA.hy926 cells with control siRNA of siRNAs specific for β-arrestin-2 (I) or Dvl-2 (J), cells were treated with thrombin (2 nM), APC (20 nM), or thrombin (2 nM) + PC-S195A (50 nM) for 3 hours. Next, cells were treated with the apoptosis inducer staurosporine (1 µM) for 8 hours. Attached and viable cells were stained with crystal violet. *P < .05 as compared with TNF-α in panel A and LPS in panel E.
EPCR occupancy inhibits the adhesion of THP-1 cells to cytokine-activated EA.hy926 endothelial cells through Dvl-2 and β-arrestin-2 biased signaling. (A-C) TNF-α (10 ng/mL)–mediated adherence of THP-1 cells to EA.hy926 cell monolayers was analyzed after treating monolayers with APC (20 nM) or thrombin (2 nM) + PC-S195A (50 nM) after transfecting cells with control siRNA (A), β-arrestin-2 siRNA (B), or Dvl-2 siRNA (C). (D) Western blot analysis of the efficiency of silencing by the control siRNA or the siRNA specific for Dvl-2. (E-G) The same as panels A-C except that the adherence of THP-1 cells to LPS (10 ng/mL)–stimulated endothelial cells were monitored. (H) Western blot analysis of the efficiency of silencing by the control siRNA or the siRNA specific for β-arrestin-2. (I-J) Analysis of EPCR-dependent cytoprotective activity of APC and thrombin by the clonogenic assay. Following transfection of EA.hy926 cells with control siRNA of siRNAs specific for β-arrestin-2 (I) or Dvl-2 (J), cells were treated with thrombin (2 nM), APC (20 nM), or thrombin (2 nM) + PC-S195A (50 nM) for 3 hours. Next, cells were treated with the apoptosis inducer staurosporine (1 µM) for 8 hours. Attached and viable cells were stained with crystal violet. *P < .05 as compared with TNF-α in panel A and LPS in panel E.
EPCR occupancy recruits GRK5 to plasma membrane
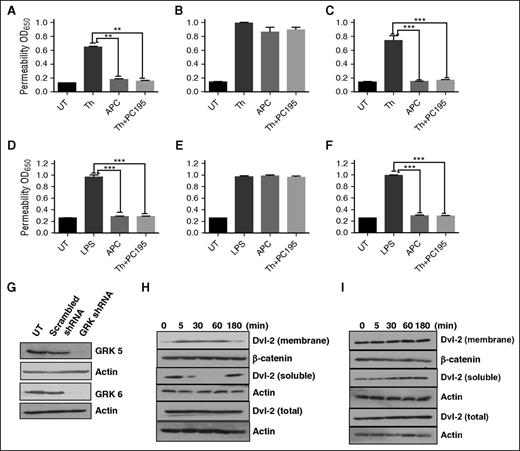
Phosphorylation of GPCRs by GRKs is thought to be responsible for phosphorylation-dependent recruitment of β-arrestins to cytoplasmic tails of receptors, thereby blocking G-protein coupled receptor signaling.32,33 Among GRK1–GRK7 members of this family of Ser/Thr kinases, only GRK5 and GRK6 can be constitutively localized and/or recruited to membrane because of their palmitoylation of specific Cys residues.33 To identify the GRK isoform, responsible for β-arrestin-2 biased PAR1 signaling by proteases, we transfected cells with a vector harboring either scrambled shRNA or shRNAs for GRK5 and GRK6. We discovered that although nonspecific shRNA had no inhibitory effect on the barrier-protective activity of APC and thrombin + PC-S195A (Figure 4A), GRK5 shRNA (Figure 4B), but not GRK6 shRNA (Figure 4C), effectively inhibited the barrier-protective activity of both proteases. This was true whether the permeability was induced with a high concentration of thrombin (Figure 4A-C) or with LPS (Figure 4D-F). The efficiency of shRNA-mediated silencing of GRK5 and GRK6 is presented in Figure 4G. Analysis of redistribution of cytosolic and membrane levels of Dvl-2 suggested that although scrambled shRNA did not change thrombin + PC-S195A–mediated enhancement of the membrane fraction of Dvl-2 (Figure 4H), GRK5 shRNA inhibited translocation of Dvl-2 from the cytoplasm to the membrane (Figure 4I). These results suggest that occupancy of EPCR is responsible for recruitment of GRK5 to cytoplasmic domain of PAR1, thereby inducing β-arrestin-2 biased signaling.
EPCR occupancy regulates β-arrestin-2 biased PAR1 signaling by recruiting GRK5. Confluent EA.hy926 endothelial cells were transfected with scrambled shRNA (A) or shRNA specific for GRK5 (B) and GRK6 (C) before treating cells with the PAR1 agonists (2 nM thrombin, 20 nM APC, or 2 nM thrombin + 50 nM PC-S195A for 3 hours) and monitoring cell permeability in response to either thrombin (10 nM for 10 minutes in panels A-C) or to LPS (10 ng/mL for 4 hours in panels D-F) as described in “Materials and methods.” (G) Western blot analysis of the efficiency of silencing by the scrambled control shRNA or shRNAs specific for GRK5 and GRK6. (H-I) Western blot analysis of Dvl-2 in cell lysates using an anti-Dvl-2 antibody in the control shRNA (H) or GRK5 shRNA silenced endothelial cells treated with thrombin (2 nM) in the absence and presence of PC-S195A (50 nM). All results are shown as means ± standard deviation of 3 different experiments. **P < .01; ***P < .001.
EPCR occupancy regulates β-arrestin-2 biased PAR1 signaling by recruiting GRK5. Confluent EA.hy926 endothelial cells were transfected with scrambled shRNA (A) or shRNA specific for GRK5 (B) and GRK6 (C) before treating cells with the PAR1 agonists (2 nM thrombin, 20 nM APC, or 2 nM thrombin + 50 nM PC-S195A for 3 hours) and monitoring cell permeability in response to either thrombin (10 nM for 10 minutes in panels A-C) or to LPS (10 ng/mL for 4 hours in panels D-F) as described in “Materials and methods.” (G) Western blot analysis of the efficiency of silencing by the scrambled control shRNA or shRNAs specific for GRK5 and GRK6. (H-I) Western blot analysis of Dvl-2 in cell lysates using an anti-Dvl-2 antibody in the control shRNA (H) or GRK5 shRNA silenced endothelial cells treated with thrombin (2 nM) in the absence and presence of PC-S195A (50 nM). All results are shown as means ± standard deviation of 3 different experiments. **P < .01; ***P < .001.
Analysis of PAR1 cleavage specificity of APC and thrombin ± PC-S195A in HeLa cells
Extracellular domain of PAR1 has 2 distinct P1-Arg (Arg-41 and Arg-46) cleavage sites that are differentially recognized by APC and thrombin for receptor signaling.19,20 Cytoprotective activity of APC can be mediated through APC cleaving PAR1 after either Arg-41 or Arg-46, whereas barrier-disruptive effect of thrombin is mediated through its cleavage after Arg-41 site,19,20 suggesting that β-arrestin-2 biased PAR1 signaling may be mediated via proteases recognizing 2 different P1-Arg sites. To further investigate this question, we constructed 2 PAR1 mutants in which P1-Arg in 2 cleavage sites was replaced with Ala. We introduced PAR1 constructs to HeLa cells and conducted permeability assays by APC and thrombin ± PC-S195A in transfected cells. We first investigated expression of EPCR and PAR1 in HeLa cells by a cell-based enzyme-linked immunosorbent assay (ELISA) and western blotting. Similar to endothelial cells, HeLa cells express EPCR; however, unlike endothelial cells, HeLa cells do not express PAR1 (Figure 5A-C). In agreement with HeLa cells not expressing PAR1, neither APC nor thrombin + PC-S195A exhibited protective effect in cells transfected with the empty vector in response to LPS (Figure 5D). Results of a cell-based assay indicated that transfection of RSV-vector harboring PAR1 complementary DNA leads to its efficient expression in HeLa cells (Figure 5E) and that both APC and thrombin + PC-S195A elicit barrier-protective effects in PAR1-transfected HeLa cells stimulated with LPS (Figure 5F). As expected and similar to endothelial cells, thrombin alone had a barrier-disruptive effect and did not exhibit cytoprotective activity in response to LPS in PAR1-WT-transfected HeLa cells (Figure 5F). These results demonstrate that we have reproduced signaling results with both APC and thrombin in HeLa cells. Next, we transfected HeLa cells with PAR1-R46A (only has Arg-41 site). Results indicated that APC cannot elicit cytoprotective effect in response to LPS in cells transfected with this construct (Figure 5G), suggesting the protective effect of APC is mediated through cleavage of Arg-46 site as has been demonstrated.19,20 Interestingly, thrombin + PC-S195A exhibited a potent barrier-protective effect in PAR1-R46A-transfected HeLa cells, suggesting thrombin cleaves after Arg-41 and that occupancy of EPCR switches PAR1 signaling specificity of thrombin to protective β-arrestin-2 biased signaling. Consistent with this hypothesis, although APC elicited a barrier-protective effect in LPS-stimulated cells transfected with PAR1-R41A (Figure 5H), thrombin + PC-S195A exhibited no protective effect (Figure 5H), supporting the hypothesis β-arrestin-2 biased PAR1 signaling by thrombin in the presence of EPCR occupancy is mediated through cleavage of Arg-41. Thus, thrombin can only cleave the receptor at Arg-41 and that it is the occupancy of EPCR that switches signaling specificity of thrombin.
Analysis of expression of PAR1 and EPCR and cytoprotective signaling in PAR1-transfected HeLa cells. (A-C) The expression of PAR1 and EPCR in EA.hy926 cells (A) or HeLa cells (B) was evaluated by a cell-based ELISA or western blotting (C) using specific antibodies as described in “Materials and methods.” (D-H) Cell permeability in response to LPS (10 ng/mL) in HeLa cells transfected with PAR1 constructs. Confluent HeLa cells were transfected with the empty vector (D) or vector containing PAR-WT (F), PAR1-R46A (G), or PAR1-R41A (H), followed by treatment of cells with PAR1 agonists (20 nM APC, 2 nM thrombin alone, or 2 nM thrombin + 50 nM PC-S195A). Cells were then treated with LPS (10 ng/mL), and the permeability was monitored as described previously. (E) The efficiency of PAR1-WT transfection in HeLa cells was monitored by a cell-based ELISA. *P < .05 as compared with untreated control (UT) in panels A, B, and E; **P < .01 as compared with LPS in panels F, G, and H.
Analysis of expression of PAR1 and EPCR and cytoprotective signaling in PAR1-transfected HeLa cells. (A-C) The expression of PAR1 and EPCR in EA.hy926 cells (A) or HeLa cells (B) was evaluated by a cell-based ELISA or western blotting (C) using specific antibodies as described in “Materials and methods.” (D-H) Cell permeability in response to LPS (10 ng/mL) in HeLa cells transfected with PAR1 constructs. Confluent HeLa cells were transfected with the empty vector (D) or vector containing PAR-WT (F), PAR1-R46A (G), or PAR1-R41A (H), followed by treatment of cells with PAR1 agonists (20 nM APC, 2 nM thrombin alone, or 2 nM thrombin + 50 nM PC-S195A). Cells were then treated with LPS (10 ng/mL), and the permeability was monitored as described previously. (E) The efficiency of PAR1-WT transfection in HeLa cells was monitored by a cell-based ELISA. *P < .05 as compared with untreated control (UT) in panels A, B, and E; **P < .01 as compared with LPS in panels F, G, and H.
EPCR occupancy confers anti-inflammatory effect for thrombin in vivo
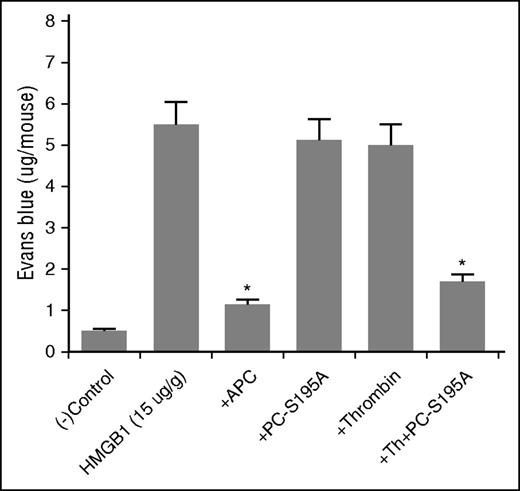
To determine whether EPCR occupancy-dependent cytoprotective function of thrombin has in vivo significance, we analyzed barrier-protective activity of thrombin in mice with or without intraperitoneal injection of PC-S195A followed by administration of thrombin as described.22 APC exhibited barrier-protective effect in response to high mobility group box 1 (HMGB1) (Figure 6), which we have shown to possess a potent hyperpermeability function in this model.22 Thrombin alone exhibited no barrier-protective effect (Figure 6). However, administration of PC-S195A prior to thrombin protected against proinflammatory effect of HMGB1, similar to that observed with APC (Figure 6). PC-S195A alone exhibited no protective function. Taken together, these results point toward in vivo relevance of our findings that EPCR occupancy endows PAR1-dependent cytoprotective effect for thrombin, although the protease is cleaving PAR1 at the P1-Arg site that is different from that of APC.
In vivo analysis of the effect of thrombin in the absence and presence of PC-S195A on vascular leakage in response to HMGB1. Mice (n = 5, for every experiment) were given intraperitoneal injection of the proteases (100 µg/kg APC and 5 nM thrombin ± 50 µg/kg PC-S195A) in 200 µL normal saline. After 30 minutes, all mice were intravenously injected with 1% bovine serum albumin–bound Evans blue dye followed by an immediate intraperitoneal injection of HMGB1 (15 µg/g body weight). Vascular permeability was determined from the extent of extravasation of Evans blue to the peritoneal cavity as described in “Materials and methods.” *P < .05 for APC as compared with HMGB1 and for thrombin + PC-S195A as compared with thrombin only and PC-S195A only.
In vivo analysis of the effect of thrombin in the absence and presence of PC-S195A on vascular leakage in response to HMGB1. Mice (n = 5, for every experiment) were given intraperitoneal injection of the proteases (100 µg/kg APC and 5 nM thrombin ± 50 µg/kg PC-S195A) in 200 µL normal saline. After 30 minutes, all mice were intravenously injected with 1% bovine serum albumin–bound Evans blue dye followed by an immediate intraperitoneal injection of HMGB1 (15 µg/g body weight). Vascular permeability was determined from the extent of extravasation of Evans blue to the peritoneal cavity as described in “Materials and methods.” *P < .05 for APC as compared with HMGB1 and for thrombin + PC-S195A as compared with thrombin only and PC-S195A only.
Discussion
In this study, we investigated EPCR- and PAR1-dependent signaling specificity of APC and thrombin and demonstrated that when EPCR is occupied both proteases elicit cytoprotective responses through β-arrestin-2 biased PAR1 signaling. Furthermore, we discovered this mechanism of PAR1 signaling is mediated by the ligated-EPCR recruiting GRK5 and Dvl-2 to the cell membrane, thereby eliciting a cytoprotective response independent of the protease (APC or thrombin) cleaving PAR1 at alternative Arg-41 or Arg-46 recognition sites. This was evidenced by the observation that thrombin + PC-S195A cleaved PAR1 at Arg-41 recognition site, yet elicited β-arrestin-2 biased cytoprotective signaling similar to that observed with APC. These results indicate the occupancy of EPCR is responsible for the β-arrestin-2 biased PAR1 signaling by both APC and thrombin even though the proteases cleave at different Arg recognition sites.
PAR1 can signal via coupling to different members of the G-protein subfamilies including Gi, Gq, and G12/13.25,26,34 It has been hypothesized that thrombin disrupts endothelial barrier function through activation of PAR1 coupled to Gq and/or G12/13.25,26,34 Interesting recent findings have indicated thrombin disrupts endothelial barrier function through activation of PAR1 at Arg-41 cleavage site,19 presumably leading to recruitment of Gq and/or G12/13 subfamily of G-proteins to the cytoplasmic domain of the receptor. By contrast, APC can cleave at the alternative Arg-46 recognition site of PAR1,19,20 thereby eliciting a cytoprotective response through β-arrestin-2 biased signaling independent of a G-protein. It has been discovered in this mechanism of GPCR signaling transmission of the signal by activated receptor is not mediated through one of the heterotrimeric G-proteins, but rather via biased signaling through one of the β-arrestins.18-20 This mechanism of GPCR signaling is initiated when cytoplasmic domain of GPCR is phosphorylated by GRKs, thereby preventing interaction of G-proteins with the receptor and favoring its interaction with β-arrestins.32,33 The mechanism by which APC but not thrombin can induce β-arrestin-2 biased PAR1 signaling is not known. Based on our previous results that occupancy of EPCR by Gla-domain of protein C/APC switches PAR1-dependent signaling specificity of thrombin from a proinflammatory to an anti-inflammatory response,21,29 we hypothesized that EPCR occupancy is responsible for the biased PAR1 signaling by recruiting a GRK to the membrane microenvironment where PAR1 signaling occurs. Among the 7 members of known GRKs, only GRK5 and GRK6 may be recruited to membrane because of their palmitoylation,32,33 which can anchor the kinases to lipid rafts/caveolae where EPCR and PAR1 are colocalized.15 To test this hypothesis, we first evaluated the membrane association of β-arrestins with the receptors by immunoprecipitation of the total protein extract of endothelial cells with anti-β-arrestin-1 and 2 antibodies followed by immunoblotting using different anti-receptor antibodies. The results indicated both β-arrestin-1 and 2 are associated with EPCR, PAR1, and caveolin-1 and that EPCR occupancy confers a PAR1-dependent cytoprotective function for thrombin through β-arrestin-2 biased signaling similar to that observed for APC. Further silencing studies revealed GRK5 is the kinase responsible for the recruitment of β-arrestin-2 to the cytoplasmic domain of PAR1 because shRNA for GRK5 specifically eliminated EPCR- and PAR1-dependent cytoprotective function of both APC and thrombin. These results suggest EPCR is responsible for β-arrestin-2 biased PAR1 signaling by both proteases.
Another interesting finding of this study was that the cytoprotective EPCR occupancy-dependent β-arrestin-2 biased PAR1 signaling by thrombin, similar to APC, facilitated recruitment of Dvl-2 to the membrane. Dvl-2 is a scaffolding protein in canonical and noncanonical Wnt signaling pathways.34,35 In the former pathway, Dvl-2 regulates Wnt signaling by activating cell surface Frizzled and LRP5/6 receptors to inactivate GSK-3β, leading to stabilization and accumulation of β-catenin in the cytoplasm. In the noncanonical pathway, Dvl-2 binds to Frizzled receptors independent of LRP5/6 to regulate the activation of Rho family of small GTPases, which are involved in modulating cytoskeleton proteins including actin and myosin.35,36 It is known that thrombin activation of PAR1 increases the level of phosphorylated myosin light chain through activation of RhoA, thereby inducing formation of actin stress fibers that can lead to cellular contraction and disruption of endothelial barrier function.25,30,31 On the other hand, barrier-protective effect of APC is mediated through APC activation of another member of the Rho family of GTPases called Rac1, which can oppose effects of RhoA in the cytoskeletal remodeling, thereby protecting endothelial cells from the edemagenic effects of thrombin and proinflammatory cytokines.25,30,31 We have previously demonstrated the occupancy of EPCR by protein C renders thrombin a potent activator of Rac1 and inhibitor of RhoA in LPS- or TNF-stimulated endothelial cells.31 Thus, in addition to β-arrestin-2, the EPCR occupancy-dependent activation of PAR1 by thrombin (and APC) recruits Dvl-2 to the plasma membrane, thereby eliciting a cytoprotective effect through modulation of the noncanonical Wnt/Frizzled/Dvl-2 signaling pathway that is independent of β-catenin. The observation that β-catenin level in all APC-mediated and thrombin + PC-S195A–mediated cytoprotective assays remained constant supports the hypothesis that these proteases activate the noncanonical Wnt signaling independent of β-catenin.
Previous results have indicated thrombin and APC can cleave at 2 different Arg-41 and Arg-46 recognition sites of PAR1 exodomain in order to elicit paradoxical barrier-disruptive and barrier-protective responses, respectively.19,20 Our results in PAR1-transfected HeLa cells, which do not express detectable levels of native PAR1, support those findings because APC elicited a protective response only in cells transfected with a PAR1 construct possessing an intact Arg-46 site. In agreement with previous results, thrombin only elicited a PAR1-dependent signaling response in cells transfected with the PAR1 construct in which Arg-41 was intact. Interestingly, the occupancy of EPCR did not change the P1-Arg cleavage specificity of PAR1 because thrombin + PC-S195A cleaved after Arg-41 to elicit a cytoprotective response, the same response APC elicits through cleavage of Arg-46 recognition site. These results support the hypothesis that coreceptor signaling by EPCR may solely be responsible for β-arrestin-2 biased PAR1 signaling and that the new N termini generated by either APC or thrombin, as the result of these proteases cleaving PAR1 exodomain at 2 different sites, can induce biased cytoprotective signaling. It is of interest to note that consistent with previous results that APC can also cleave after Arg-41,19 APC reproducibly exhibited a very modest (although not statistically significant) cytoprotective response in cells transfected with PAR1-R46A (Figure 5G). This observation is in line with our hypothesis that signaling through either Arg-41 or Arg-46 sites are protective as long as EPCR is occupied by the Gla-domain of protein C. Based on results presented here and on our previous data, we propose the following model (Figure 7) for the mechanism of PAR1 signaling by APC and thrombin. When EPCR is not occupied by its ligand, the activation of PAR1 by thrombin couples the receptor to Gq and/or G12/13 proteins to activate the RhoA GTPase, leading to barrier-disruptive response in endothelial cells. We previously demonstrated EPCR is associated with caveolin-1 in the membrane lipid rafts under these conditions.15,21 When EPCR is occupied by its ligand, EPCR dissociates from caveolin-1, and this process is somehow linked to recruitment of GRK5, β-arrestin-2, and Dvl-2 to the plasma membrane where PAR1 is located. These events prevent coupling of the Gα subunit of the heterotrimeric G-proteins to the PAR1 C-terminal tail, thus signaling proceeds through β-arrestin-2 biased signaling mechanism. It is of interest to note β-arrestin-2 biased protective signaling function of both APC and thrombin + PC-S195A was also found to require receptor signaling through a Gi-protein as evidenced by the PTX (Gi-protein specific inhibitor) inhibiting this response. This is consistent with the literature that PAR1-dependent protective activity of APC also requires cross talk with S1P1, which is a known Gi-protein coupled receptor.28,30 In support of this hypothesis, S1P1 silencing eliminated protective responses of both APC and thrombin + PC-S195A in LPS-stimulated endothelial cells. One study has reported a Gi-protein-independent protective signaling activity for APC.18 However, the experimental approach in that study was not monitoring cytoprotective activity of APC in a functional assay.18
Hypothetical model of EPCR-dependent PAR1 signaling by APC and thrombin in endothelial cells. When EPCR is not ligated by protein C/APC, EPCR is associated with caveolin-1 in lipid rafts of endothelial cells.21 In this case, thrombin cleavage of PAR1 after Arg-41 recognition site couples the receptor to Gq and/or G12/13, thereby enhancing the cellular permeability through the activation of RhoA GTPase and NF-κB signaling pathways. When EPCR is occupied by protein C/APC, the receptor dissociates from caveolin-1,21 which is a process somehow linked to the recruitment of GRK5 to the plasma membrane. In this case, the cleavage of PAR1 by either thrombin after Arg-41 or APC after Arg-46 sites result in the GRK-dependent phosphorylation of the N-terminal cytoplasmic domain of the cleaved PAR1 and inhibition of its interaction with anyone of the Gα subunit of the heterotrimeric G proteins. The EPCR-dependent cleavage of either Arg-41 or Arg-46 sites recruits β-arrestin-2 and Dvl-2, thereby transmitting the PAR1 signal through so called “β-arrestin-2 biased” signaling mechanism. The PAR1-dependent β-arrestin-2/Dvl-2 signaling activates Rac1 GTPase, inhibits NF-κB, and increases the barrier integrity of endothelial cells. The EPCR- and PAR1-dependent cytoprotective responses of both APC and thrombin require cross talk with Gi-protein coupled S1P1 signaling. Whether this latter GPCR signaling is mediated directly via sphingosine 1-phosphate itself or mediated indirectly through the phosphorylation/activation of the cytoplasmic domain of S1P1 by an activated kinase (ie, phosphatidylinositol 3-kinase/protein kinase B [PI-3K/Akt]) is not known and requires further investigation. See the text for more details. Cav-1, caveolin-1; Exo I, exosite I; PC, protein C; S1P, sphingosine 1-phosphate.
Hypothetical model of EPCR-dependent PAR1 signaling by APC and thrombin in endothelial cells. When EPCR is not ligated by protein C/APC, EPCR is associated with caveolin-1 in lipid rafts of endothelial cells.21 In this case, thrombin cleavage of PAR1 after Arg-41 recognition site couples the receptor to Gq and/or G12/13, thereby enhancing the cellular permeability through the activation of RhoA GTPase and NF-κB signaling pathways. When EPCR is occupied by protein C/APC, the receptor dissociates from caveolin-1,21 which is a process somehow linked to the recruitment of GRK5 to the plasma membrane. In this case, the cleavage of PAR1 by either thrombin after Arg-41 or APC after Arg-46 sites result in the GRK-dependent phosphorylation of the N-terminal cytoplasmic domain of the cleaved PAR1 and inhibition of its interaction with anyone of the Gα subunit of the heterotrimeric G proteins. The EPCR-dependent cleavage of either Arg-41 or Arg-46 sites recruits β-arrestin-2 and Dvl-2, thereby transmitting the PAR1 signal through so called “β-arrestin-2 biased” signaling mechanism. The PAR1-dependent β-arrestin-2/Dvl-2 signaling activates Rac1 GTPase, inhibits NF-κB, and increases the barrier integrity of endothelial cells. The EPCR- and PAR1-dependent cytoprotective responses of both APC and thrombin require cross talk with Gi-protein coupled S1P1 signaling. Whether this latter GPCR signaling is mediated directly via sphingosine 1-phosphate itself or mediated indirectly through the phosphorylation/activation of the cytoplasmic domain of S1P1 by an activated kinase (ie, phosphatidylinositol 3-kinase/protein kinase B [PI-3K/Akt]) is not known and requires further investigation. See the text for more details. Cav-1, caveolin-1; Exo I, exosite I; PC, protein C; S1P, sphingosine 1-phosphate.
Finally, as demonstrated in vivo, we propose that the EPCR occupancy-dependent cytoprotective PAR1 signaling by thrombin has pathophysiological significance for the maintenance of the vascular tone during the activation of coagulation. We hypothesize that thrombin not only initiates the anticoagulant and anti-inflammatory APC pathway by activation of the EPCR-bound protein C in lipid rafts of endothelial cells, but can also directly elicit anti-inflammatory responses through activation of PAR1 in the same microenvironment. This hypothesis contrasts with the traditional view that cleavage of PAR1 by thrombin is barrier disruptive in the vasculature. Our hypothesis envisions that the cleavage of PAR1 by thrombin may be proinflammatory only in cells lacking EPCR or when EPCR is downregulated and/or missing because of injury-mediated denudation of the vessel wall endothelial cells, in particular if PAR1 positive and EPCR negative cells of subendothelial layer are exposed. It is of interest to note that EPCR occupancy by APC has also been found to inhibit proinflammatory PAR2 signaling by the ternary tissue factor/factor VIIa/factor Xa complex, suggesting a critical role for EPCR in regulating the PAR-dependent signaling specificity of coagulation proteases.37
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors would like to thank Audrey Rezaie for editorial work on the manuscript.
This work was supported by grants from the National Institutes of Health National Heart, Lung, and Blood Institute (HL101917 and HL62565) (A.R.R.).
Authorship
Contribution: R.V.R. and A.A. designed experiments, analyzed data, and performed research; P.D. conducted animal experiments; L.Y. constructed the PAR1 mutants; and A.R.R. designed experiments, analyzed data, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Alireza R. Rezaie, Edward A. Doisy Department of Biochemistry and Molecular Biology, Saint Louis University School of Medicine, 1100 S. Grand Blvd, St. Louis, MO 63104; e-mail: rezaiear@slu.edu.
References
Author notes
R.V.R. and A.A. contributed equally to this study.

![Figure 7. Hypothetical model of EPCR-dependent PAR1 signaling by APC and thrombin in endothelial cells. When EPCR is not ligated by protein C/APC, EPCR is associated with caveolin-1 in lipid rafts of endothelial cells.21 In this case, thrombin cleavage of PAR1 after Arg-41 recognition site couples the receptor to Gq and/or G12/13, thereby enhancing the cellular permeability through the activation of RhoA GTPase and NF-κB signaling pathways. When EPCR is occupied by protein C/APC, the receptor dissociates from caveolin-1,21 which is a process somehow linked to the recruitment of GRK5 to the plasma membrane. In this case, the cleavage of PAR1 by either thrombin after Arg-41 or APC after Arg-46 sites result in the GRK-dependent phosphorylation of the N-terminal cytoplasmic domain of the cleaved PAR1 and inhibition of its interaction with anyone of the Gα subunit of the heterotrimeric G proteins. The EPCR-dependent cleavage of either Arg-41 or Arg-46 sites recruits β-arrestin-2 and Dvl-2, thereby transmitting the PAR1 signal through so called “β-arrestin-2 biased” signaling mechanism. The PAR1-dependent β-arrestin-2/Dvl-2 signaling activates Rac1 GTPase, inhibits NF-κB, and increases the barrier integrity of endothelial cells. The EPCR- and PAR1-dependent cytoprotective responses of both APC and thrombin require cross talk with Gi-protein coupled S1P1 signaling. Whether this latter GPCR signaling is mediated directly via sphingosine 1-phosphate itself or mediated indirectly through the phosphorylation/activation of the cytoplasmic domain of S1P1 by an activated kinase (ie, phosphatidylinositol 3-kinase/protein kinase B [PI-3K/Akt]) is not known and requires further investigation. See the text for more details. Cav-1, caveolin-1; Exo I, exosite I; PC, protein C; S1P, sphingosine 1-phosphate.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/128/14/10.1182_blood-2016-06-720581/4/m_1884f7.jpeg?Expires=1765185205&Signature=QlWvLb8OHhq9YqCC1KlRC0Vl8Kc-gmyy65BKQk-c~m5ujfqGqkwiaLNvnmc2PAcc6Odd0zeq7zoIjzXyvrOwswoRgJ7uz48v1UTmpBVQ~DfTcXr39zG68x9BxsIMYfBUqoKjtwCifWbROuvWB9UVT41J16FhzLwfTXbPY~Aljh6VPa2caAMXnDUS60l5GzRUssShps7EK5wsq4s~2-TTojmoLXHvWS~iSV6JEYk7oUqLIP8farRTDnnvZzViRhJVdTI0ewu7owT9Q6QXTQ2M~47-Jj5mdPsaFPECjnG35ESH5VoXEeR5yT~qOWszk6JTBZe9rrzQc9Fae-GvSd~Jiw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
