In this issue of Blood, Avila et al provide valuable information on outcomes and important predictive factors relevant to lower extremity deep vein thrombosis (LE-DVT) in children.1
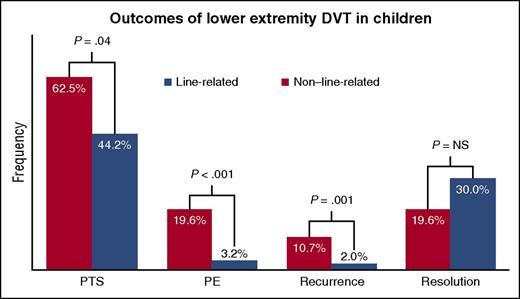
Outcomes reported for 339 children with LE-DVT (283 with line-related, 56 with non–line-related). NS, not significant.
Outcomes reported for 339 children with LE-DVT (283 with line-related, 56 with non–line-related). NS, not significant.
Although rarer than in adults, DVT is a growing problem in pediatrics,2 as are its complications, which include postthrombotic syndrome (PTS), pulmonary embolism (PE), recurrence, and lack of thrombus resolution. However, in contrast to the growing evidence base for the disease in adults,3 even basic knowledge regarding the frequency of such outcomes is sparse for pediatric DVT; available information is limited mostly to registry and case series data.2
The study by Avila et al advances the knowledge base in this arena through analysis of a large pediatric cohort (n = 339) with LE-DVT, followed clinically every 18 months per institutional protocol. This extended follow-up included careful assessment for PTS, evaluable data for which were available for >98% of eligible patients. In children, 2 of the most significant risk factors for DVT occurrence are neonatal age and use of a central venous catheter.2 The authors analyzed the data according to these highly distinctive parameters and found that the minority of patients who developed thrombosis unrelated to catheter use (non–line-related [non-LR]) exhibited higher rates of PTS, PE, and clot recurrence than those with line-related thrombosis (LR). Although not statistically significant, the frequency of thrombus resolution was also lower in the non-LR group (see figure). Multivariate analyses identified the presence of any occlusive segment at diagnosis as a predictor for lack of thrombus resolution by the end of therapy, which in turn was a predictor for the longer-term sequela, PTS. Sex and LR/non-LR status interacted as predictors for PTS. Although the analyses differed slightly, a similar study from the same institution profiling patients with upper extremity DVT also found that secondary DVT events (which were mostly LR) resulted in lower rates of PTS and implicated residual thrombus as a risk factor for PTS development.4
Collectively, these findings provide important baseline outcome data for a condition that is more and more commonly encountered in clinical practice. Moreover, the results stress the importance of ascertaining the relevant risk factors contributing to incident DVT in children, as LR/non-LR status conveys rich clinical information, including that LR DVT generally portends more favorable outcomes compared with non-LR DVT. From the standpoint of clinical trial design in pediatric DVT, the study also offers validation for clot resolution as a surrogate end point for PTS.
Other important clinical observations from the study deserve special mention. For example, children who developed thrombosis in the absence of a central venous catheter (non-LR) were highly likely to have an underlying predisposing condition, despite this not being apparent at the time of LE-DVT diagnosis (true for 61% of the non-LR group). Of course, such conditions included plasma-based thrombophilic traits, which were assessed in 95% of the non-LR group (importantly, thrombophilia was not predictive of either PTS or thrombus resolution). Beyond these traits, however, about three quarters of patients lacking an overt underlying condition at LE-DVT diagnosis were eventually diagnosed with a rheumatologic disorder, inflammatory bowel disease, or a venous anatomic variant. In particular, appropriate suspicion for the latter class of conditions, which comprised May-Thurner syndrome and inferior vena cava anomalies in this cohort, is critical for optimizing management, as evidenced by the significant recurrence rate (17%) among patients with variants. Such patients may warrant consideration for secondary thromboprophylaxis via indefinite anticoagulation.
Additionally, PTS, as defined by a Modified Villalta Scale (MVS) score >1, was very common; nearly half of patients in the cohort met this criterion. PTS onset and progression were confirmed to be indolent, over the course of years, with earliest onset occurring in the non-LR group, for which median time to detection was 2.0 years after diagnosis compared with 10.5 years for the neonatal LR group. Notably, >95% of cases were classified as mild by the MVS classification, with no cases of severe PTS or skin ulceration. How clinically significant were these cases of so-called “mild” PTS? On the one hand, some evidence suggests that mild PTS may not measurably detract from quality of life.5 Conversely, the possibility that the MVS may not adequately discriminate PTS that is clinically important6 or that does impact meaningfully on quality of life, should be considered. For example, the MVS does not uniformly assess for severity of individual signs and symptoms of PTS but merely their presence or absence for most of the scoring components. To illustrate, a child with severe chronic pain or swelling from PTS could still exhibit an MVS score that tallies as only “mild” despite the significant symptom severity. Despite this uncertainty, clinicians treating patients for LE-DVT must consider, based on this study’s findings, whether regular follow-up over several years should become routine clinical practice. Many likely do not follow patients regularly after the anticoagulation course is complete, let alone as often as every 18 months until transfer to adult care, which was the standard institutional practice for this cohort.
The study results highlight additional areas deserving further attention. In the final analysis, the apparent lack of association between more intensive treatment (ie, thrombolysis) and PTS outcome in this cohort is notable in light of limited pediatric data and the more extensive adult experience supporting the efficacy of catheter-directed thrombolysis in reducing PTS risk.7,8 Results from completed adult (#NCT00790335) and planned pediatric (#NCT02767232) trials are expected to provide more definitive guidance on this point. Furthermore, the high rate of inadequate thrombus resolution (>25% of patients with clot extension or no resolution) underscores the need for identifying more effective DVT treatment approaches. Finally, a standardized, well-validated PTS assessment tool that can easily incorporate into clinical practice is another need. The ideal tool will identify clinically important PTS and track severity of distinct signs and symptoms over time.9 Such future development requires concurrent quality of life assessment using appropriate instruments5 and studies of the economic impact of PTS, so that the effects of this potentially burdensome disorder can be more completely understood in children.
Conflict-of-interest disclosure: D.L.Y. is a local principal investigator and serves on steering committees on anticoagulant clinical trials funded by the pharmaceutical industry, including Bristol-Myers Squibb, Pfizer, and Daiichi Sankyo.