Key Points
Inhibiting endosomal TLRs suppresses MYD88L265P B-cell proliferation in vitro.
Inhibition of endosomal TLRs paradoxically enhances accumulation of MYD88L265P B cells as plasmablasts in vivo.
Abstract
The MYD88L265P mutation is found in 2% to 10% of chronic lymphocytic leukemia, 29% of activated B-cell type diffuse large B-cell lymphoma and 90% of Waldenström macroglobulinemia, making it conceptually attractive to treat these malignancies with inhibitors of endosomal Toll-like receptors (TLR9, TLR7) that activate MYD88. Here we show that genetic inhibition of endosomal TLRs has the opposite effect on accumulation of MYD88L265P B cells in vitro and in vivo. Activated mature B cells from wild-type, Unc93b13d/3d-mutant, or Tlr9-deficient mice were transduced with retrovirus encoding MYD88L265P and analyzed either in vitro or after transplantation into Rag1−/− recipient mice. Unc93b13d/3d mutation, which blocks TLR9 and TLR7 signaling, or Tlr9 deficiency suppressed MYD88L265P B-cell growth in vitro but paradoxically increased in vivo accumulation of MYD88L265P B cells as CD19low plasmablasts by 10- to 100-fold. These results reveal an unexpected, powerful inhibitory effect of TLR9 on MYD88L265P B-cell proliferation and differentiation that appears independent of TLR7, and they provide a preclinical indicator for caution in clinical trials of TLR7/9 inhibitors for MYD88L265P B-cell malignancies.
Introduction
Many lymphomas are incurable with current regimens, making it essential to identify driver mutations that can be targeted.1 The MYD88L265P mutation occurs frequently in chronic lymphocytic leukemia, activated B-cell type diffuse large B-cell lymphoma, and Waldenström macroglobulinemia (WM).2-4 MYD88 is an adaptor protein downstream of most Toll-like receptors (TLRs) and interleukin-1 (IL-1) and IL-18 receptors.5 It contains a C-terminal Toll/IL-1 receptor domain for interacting with a range of receptors and an N-terminal death domain for recruiting IL-1 receptor–associated kinases to activate the nuclear factor κB signaling pathway. The L265P substitution in the C-terminal Toll/IL-1 receptor domain constitutively activates the TLR signaling pathway to activate nuclear factor κB, on which activated B-cell type diffuse large B-cell lymphoma and WM cell lines rely for growth and survival.3,4
We previously found that MYD88L265P is sufficient to drive spontaneous but self-limiting proliferation of primary mouse B cells in vitro and in vivo.6 MYD88L265P-induced proliferation in tissue culture was inhibited by functional deficiencies of Unc93b1 or chloroquine, which interfere with trafficking to endosomes and signaling by nucleic acid sensing TLR9 and TLR7, and by Tlr9 genetic deficiency.6 Pharmacological inhibitors of TLR9 may therefore be considered to treat MYD88L265P-bearing lymphomas and WM disease, promoting a phase 1/2 trial.7 Here we report the unexpected, important finding that interference with TLR9 signaling paradoxically promotes accumulation of MYD88L265P lymphoplasmablasts in vivo.
Study design
B-cell transduction, transplantation, flow cytometric, and serum analysis
Donor mice were either wild type, Unc93b13d/3d, or Tlr9−/− C57BL/6.8,9 B cells were activated with anti–immunoglobulin M (IgM) and anti-CD40, transduced with retroviral vectors, and washed 3 times, and ∼5 × 105 viable EGFP+ B cells (supplemental Figure 1, available on the Blood Web site) were transplanted into Rag1−/− recipient mice.10 Recipient spleen cells were analyzed by flow cytometry and serum by enzyme-linked immunosorbent assay (ELISA) 11 days later, all as previously described.6,11
Results and discussion
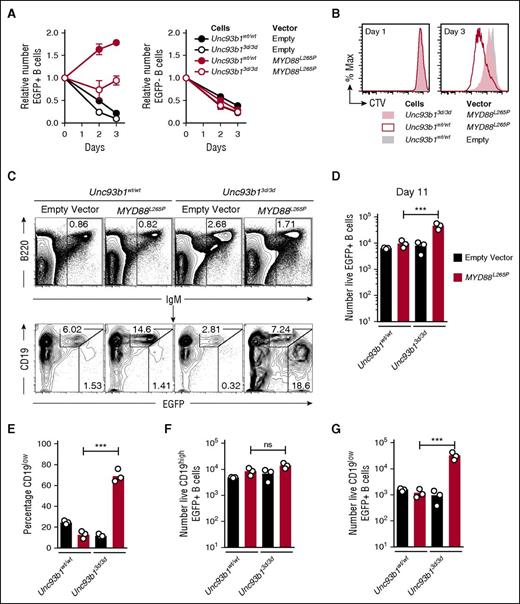
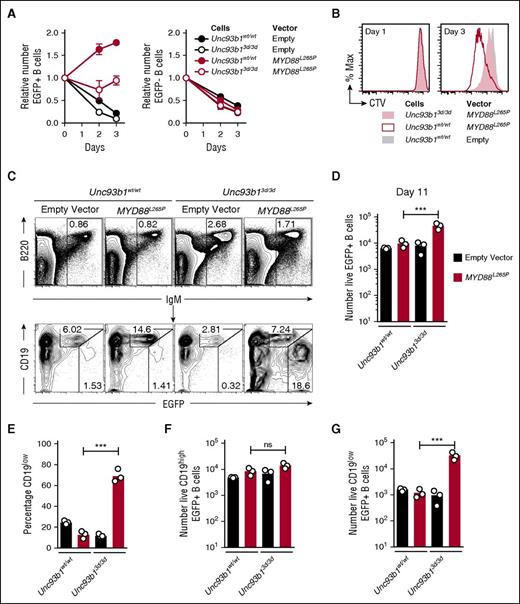
Activated splenic B cells were transduced with a bicistronic retroviral vector encoding MYD88L265P and green fluorescent protein (EGFP) or a control empty vector encoding only EGFP. As found previously,6 expression of MYD88L265P in the EGFP+ B cells was sufficient to drive multiple rounds of B-cell division when the cells were placed in tissue culture without mitogens, and this in vitro proliferation was reduced by the Unc93b13d/3d mutation (Figure 1A-B). Half of the transduced B cells were also transplanted into B-cell deficient Rag1−/− mice by intravenous injection (supplemental Figure 1A-B) and enumerated in the spleen after 11 days by flow cytometry (Figure 1C-D). Opposite to the findings in tissue culture, 500% more MYD88L265P EGFP+ cells accumulated when they were Unc93b13d/3d compared with Unc93b1wt/wt (Figure 1D). Further analysis of the transplanted EGFP+ B cells expressing MYD88L265P revealed that >65% of accumulating Unc93b13d/3d B cells but only 15% of Unc93b1wt/wt B cells adopted a state with low surface CD19 (CD19low) and high EGFP, consistent with the phenotype of cells that have undergone plasmablast differentiation (Figure 1C,E). Unc93b13d/3d mutant B cells transduced with the control EGFP only vector did not show increased CD19low plasmablast formation, indicating that the plasmablast accumulation reflected cooperation between the Unc93b13d/3d mutation and MYD88L265P (Figure 1C,E). The mean number of CD19low EGFP+ cells was increased 20-fold, whereas the number of CD19hi cells was not increased (Figure 1F-G).
Unc93b13d/3d mutation paradoxically increases accumulation of CD19lowMYD88L265P B cells in vivo. (A) Anti-IgM plus anti-CD40 activated Unc93b1wt/wt or Unc93b13d/3d B cells were transduced with the indicated vectors that also encoded EGFP, washed, and cultured in triplicate without mitogen (day 0) for 3 days. Mean and standard deviation number of EGFP+ (left) and EGFP− (right) cells were compared with the starting number on day 0 of the culture. Data are representative of 3 independent experiments. (B) Cell division measured by cell trace violet (CTV) dilution on days 1 and 3 of culture without antigen or CD40 stimulation, gated on EGFP+ cells expressing the indicated vectors. (C) Flow cytometric analysis of the spleens of Rag1−/− recipient mice 11 days after transplantation of transduced B cells. Plots show concatenated data from 3 recipients per treatment: B220 vs IgM plots show the mean percentage of live spleen lymphocytes falling within the indicated IgM+ B-cell gate; CD19 vs EGFP plots are gated on the IgM+ B cells and show the percentage of EGFP-expressing cells with either high or low levels of CD19 expression. (D) Total number of live EGFP+ cells in the spleen of each recipient mouse. (E) Percentage of CD19low cells among live EGFP+ cells in the spleen of each recipient mouse. Total number of live CD19high EGFP+ (F) and CD19low EGFP+ (G) cells in the spleen of each recipient mouse. Data are representative of 3 independent experiments. Statistical analysis by unpaired Student t-test. ***P < .001.
Unc93b13d/3d mutation paradoxically increases accumulation of CD19lowMYD88L265P B cells in vivo. (A) Anti-IgM plus anti-CD40 activated Unc93b1wt/wt or Unc93b13d/3d B cells were transduced with the indicated vectors that also encoded EGFP, washed, and cultured in triplicate without mitogen (day 0) for 3 days. Mean and standard deviation number of EGFP+ (left) and EGFP− (right) cells were compared with the starting number on day 0 of the culture. Data are representative of 3 independent experiments. (B) Cell division measured by cell trace violet (CTV) dilution on days 1 and 3 of culture without antigen or CD40 stimulation, gated on EGFP+ cells expressing the indicated vectors. (C) Flow cytometric analysis of the spleens of Rag1−/− recipient mice 11 days after transplantation of transduced B cells. Plots show concatenated data from 3 recipients per treatment: B220 vs IgM plots show the mean percentage of live spleen lymphocytes falling within the indicated IgM+ B-cell gate; CD19 vs EGFP plots are gated on the IgM+ B cells and show the percentage of EGFP-expressing cells with either high or low levels of CD19 expression. (D) Total number of live EGFP+ cells in the spleen of each recipient mouse. (E) Percentage of CD19low cells among live EGFP+ cells in the spleen of each recipient mouse. Total number of live CD19high EGFP+ (F) and CD19low EGFP+ (G) cells in the spleen of each recipient mouse. Data are representative of 3 independent experiments. Statistical analysis by unpaired Student t-test. ***P < .001.
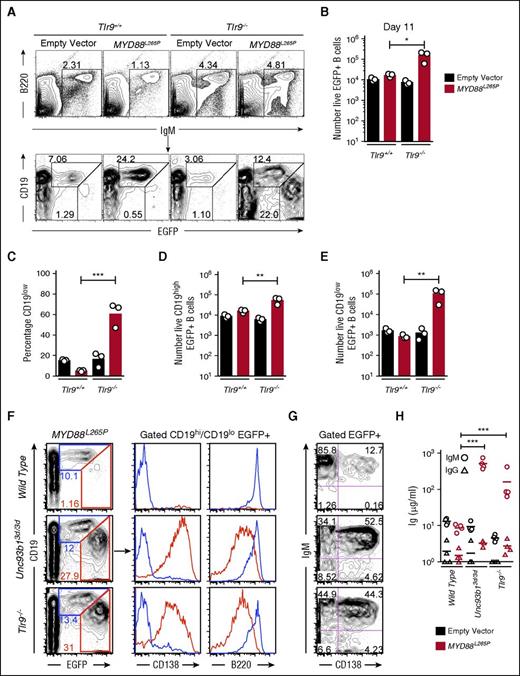
The paradoxical enhancement of MYD88L265P induced plasmablast formation by the Unc93b13d/3d mutation could reflect crippling of TLR7 and TLR9 signaling or an effect on an independent inhibitory pathway. To resolve between these possibilities, we tested if TLR9 deficiency (Tlr9−/−) could recapitulate the effects of Unc93b13d/3d mutation. Wild-type (Tlr9+/+) or Tlr9−/− B cells were transduced with empty EGFP only or MYD88L265P EGFP vectors and transplanted into Rag1−/− recipient mice in comparable numbers (supplemental Figure 1C-D). After 11 days, 60% of Tlr9−/−MYD88L265P EGFP+ B cells were CD19low plasmablasts, whereas Tlr9+/+ B cells transduced with the same MYD88L265P EGFP vector only increased in EGFP+ percentage but did not downregulate CD19 (Figure 2A,C). Tlr9−/−MYD88L265P EGFP+ B cells accumulated in 10 times greater number compared with Tlr9+/+ B cells transduced with the MYD88L265P EGFP vector (Figure 2B). This increase stemmed from 3-fold more CD19high and 100-fold more CD19low EGFP+ cells (Figure 2D-E). Further analysis showed that both Unc93b13d/3d and Tlr9−/−MYD88L265P CD19low EGFP+ cells were CD138+ B220low unswitched plasmablasts that secreted large amounts of serum IgM but little IgG (Figure 2F-H). The simplest interpretation of these data are that absence of active TLR9 accounts for the effect of the Unc93b13d/3d mutation in dramatically enhancing MYD88L265P plasmablast accumulation in vivo.
Tlr9 deficiency equally increases MYD88L265P B-cell proliferation and differentiation into IgM plasmablast in vivo. (A) Flow cytometric analysis of the spleens of Rag1−/− recipient mice 11 days after transplantation of transduced B cells. Plots show concatenated data from 3 recipients per treatment: B220 vs IgM plots show the mean percentage of live spleen lymphocytes falling within the indicated IgM+ B-cell gate; CD19 vs EGFP plots are gated on the IgM+ B cells and show the percentage of EGFP-expressing cells with either high or low levels of CD19 expression. (B) Total number of live EGFP+ cells in the spleen of each recipient mouse. (C) Percentage of CD19low cells among live EGFP+ cells in the spleen of each recipient mouse. Total number of live CD19high EGFP+ (D) and CD19low EGFP+ (E) cells in the spleen of each recipient mouse. Data are representative of 3 independent experiments. (F) Flow cytometric analysis as performed in panel A; plots show concatenated data from 4 recipients per treatment: CD19 vs EGFP plots are gated on live cells and show the percentage of EGFP expressing cells with either high or low levels of CD19 expression; histograms display CD138 and B220 expression and are gated on either CD19high or CD19low EGFP+ cells. (G) Plots show CD138 and IgM expression on live EGFP+ cells as analyzed in panel F. (H) Sera IgM and IgG measured by ELISA from groups of Rag1−/− recipient mice on the day of flow cytometric analysis. Statistical analysis by unpaired Student t-test. *P < .05; **P < .01; ***P < .001.
Tlr9 deficiency equally increases MYD88L265P B-cell proliferation and differentiation into IgM plasmablast in vivo. (A) Flow cytometric analysis of the spleens of Rag1−/− recipient mice 11 days after transplantation of transduced B cells. Plots show concatenated data from 3 recipients per treatment: B220 vs IgM plots show the mean percentage of live spleen lymphocytes falling within the indicated IgM+ B-cell gate; CD19 vs EGFP plots are gated on the IgM+ B cells and show the percentage of EGFP-expressing cells with either high or low levels of CD19 expression. (B) Total number of live EGFP+ cells in the spleen of each recipient mouse. (C) Percentage of CD19low cells among live EGFP+ cells in the spleen of each recipient mouse. Total number of live CD19high EGFP+ (D) and CD19low EGFP+ (E) cells in the spleen of each recipient mouse. Data are representative of 3 independent experiments. (F) Flow cytometric analysis as performed in panel A; plots show concatenated data from 4 recipients per treatment: CD19 vs EGFP plots are gated on live cells and show the percentage of EGFP expressing cells with either high or low levels of CD19 expression; histograms display CD138 and B220 expression and are gated on either CD19high or CD19low EGFP+ cells. (G) Plots show CD138 and IgM expression on live EGFP+ cells as analyzed in panel F. (H) Sera IgM and IgG measured by ELISA from groups of Rag1−/− recipient mice on the day of flow cytometric analysis. Statistical analysis by unpaired Student t-test. *P < .05; **P < .01; ***P < .001.
Effects of TLR9 deficiency resembling those here have been observed in lupus-prone Faslpr mutant mice, where TLR9 deficiency increases accumulation of anti-DNA follicular B cells12 and increases autoantibody production and disease severity.13-15 One explanation is that TLR9 outcompetes TLR7 for association with UNC93B1.16 In Faslpr mice, autoantibody formation in the absence of TLR9 requires TLR7 and is inhibited by combined genetic deficiency of TLR9 and TLR717 or by the Unc93b13d/3d H412R missense mutation.18-20 By contrast, the exaggerated accumulation of Unc93b13d/3d mutant plasmablasts observed here indicates that a different mechanism, independent of TLR9, TLR7, or TLR3 signaling,8 accounts for spontaneous proliferation of MYD88L265P B cells in vivo and the inhibitory effect of TLR9 in this context.
Inhibition of TLR7/8/9 signaling has been considered as therapy for lymphoproliferative diseases with the MYD88L265P mutation. An inhibitory oligonucleotide is currently under evaluation in a phase 1/2 clinical trial in patients with relapsed or refractory WM.7 These inhibitors have also shown promising results for the suppression of cell growth in tumor cell lines and in vivo xenografts of MYD88L265P positive lymphomas.21 However, the preclinical observation here of exaggerated in vivo accumulation of MYD88L265P plasmablasts caused by genetic inhibition of TLR9 alone or combined inhibition of TLR9/7/3 raises the possibility that inhibiting these receptors in patients may also paradoxically increase accumulation of malignant B cells bearing the MYD88L265P mutation, although we recognize that malignant transformation may modify the phenomena observed here.
Proliferation and differentiation of MYD88L265P B cells in vivo could be stimulated by a range of other receptors that activate MYD88 independently of UNC93B1 and recognize ligands present in the recipient mice for transplantation experiments but not in tissue culture. For example, in vivo growth of MYD88L265P B cells could be stimulated by lipopolysaccharides or flagellin from bacterial flora through TLR4 or TLR5, respectively, or by the cytokines IL-1 and IL-18.22 The tumor necrosis factor family B-cell cytokines, B-cell activating factor (BAFF) and a proliferation-inducing ligand (APRIL), also activate MYD88 in B cells through transmembrane activator and calcium-modulating cyclophilin ligand interactor.23 Interestingly, WM patients generally express higher levels of BAFF than healthy controls, leading to phase 1 trials of soluble transmembrane activator and calcium-modulating cyclophilin ligand interactor–Ig fusion protein as a BAFF/APRIL inhibitor in WM.24,25
In sum, our findings reveal a much more complex interplay between MYD88L265P and TLRs in controlling B-cell proliferation and differentiation, with potentially beneficial or adverse implications for different pathway-directed therapeutics in B-cell malignancies.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Paula Gonzalez for advice on ELISA and the Australian Phenomics Facility for expert care and genotyping of animals.
This work was supported by grants from the National Institutes of Health, National Institute of Allergy and Infectious Diseases (AI100627) (C.C.G., B.B.) and the National Health and Medical Research Council (1016953, 585490, and 1081858 [C.C.G.]; 1086770 [K.H.]). J.Q.W is a candidate at the Australian National University and is supported by an Australian Postgraduate Award.
Authorship
Contribution: J.Q.W. designed and performed experiments, analyzed results, made figures, and wrote the manuscript; B.B. provided the Unc93b13d/3d mutant mice and advised experimental design and interpretation; and C.C.G. and K.H. supervised the research and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Christopher C. Goodnow, Immunogenomics Laboratory, Immunology Division, Garvan Institute of Medical Research, 384 Victoria St, Darlinghurst, NSW 2010, Australia; e-mail: c.goodnow@garvan.org.au; and Keisuke Horikawa, Australian Cancer Research Foundation (ACRF) Department of Cancer Biology and Therapeutics, The John Curtin School of Medical Research, 131 Garran Rd, Acton, ACT 2601, Australia; e-mail: keisuke.horikawa@anu.edu.au.
References
Author notes
C.C.G. and K.H. contributed equally to this study.