Abstract
Platelets are essential components of hemostasis. Due to a plethora of factors released on activation, platelet functions are also connected to tumor growth, notably by acting on angiogenesis. It is now well recognized that major roles of platelets in the poor outcome of cancer patients occurs during hematogenous dissemination of cancer cells. In this review, we describe recent insights into the molecular mechanisms supporting the prometastatic activity of platelets. Platelets have been shown to promote survival of circulating tumor cells (CTCs) in the bloodstream by conferring resistance to the shear stress and attack from natural killer cells. Recently, platelets were found to promote and/or maintain the state of epithelial to mesenchymal transition on CTCs through platelet secretion of transforming growth factor β in response to CTC activation. At a later stage in the metastatic process, platelets promote extravasation and establishment of metastatic cells in distant organs as observed in bone. This particular environment is also the site of hematopoiesis, megakaryocytopoiesis, and platelet production. Increasing the number of megakaryocytes (MKs) in the bone marrow results in a high bone mass phenotype and inhibits skeletal metastasis formation of prostate cancer cells. As a result of their specific location in vascular niches in the bone marrow, MK activity might contribute to the “seed and soil” suitability between CTCs and bone. In conclusion, recent findings have made a great advance in our knowledge on how platelets contribute to the metastatic dissemination of cancer cells and that may support the development of new antimetastasis therapies.
Platelets in tumor growth and angiogenesis
Besides their vital role in the maintenance of blood barrier integrity and hemostasis, platelets express cell surface molecules and secrete a plethora of proteins, nucleotides, and bioactive lipids that also promote inflammation and cancer progression (Table 1).1,2 Secondary development of metastases at distant sites from the primary tumor involves complex mechanisms starting with altered cell adhesion, survival, invasion (migration/proteolysis), and access to blood or lymphatic vessels allowing systemic dissemination of tumor cells. Increased angiogenesis provides better access of these cells to the blood stream favoring metastasis dissemination that gains through the assistance of platelets.3 The hypothesis that platelets may control tumor angiogenesis was developed 2 decades ago based on the rationale that they are a rich source of angiogenesis modulators and they interact with the endothelium.4 Cancer cell–induced platelet activation was shown in particular to induce the release of proteases and bioactive phospholipids that promote angiogenesis.5 Platelet adhesive molecules might also play an important role in modulating angiogenesis, as found recently using P-selectin–deficient platelets and soluble P-selectin, which abolished platelet deposition within the tumors by decreasing vascular endothelial growth factor (VEGF) secretion, angiogenesis, and thereby suppressing tumor growth.6 However, the role of platelets in angiogenesis is not fully understood because their granular contents eventually secreted on aggregation release a wild range of both pro- VEGF, platelet-derived growth factor [PDGF], fibroblast growth factor-2) and antiangiogenic factors (endostatin, thrombospondin-1, platelet factor 4, plasminogen activation inhibitor-1) (Table 1). Platelet degranulation is often presented as a burst resulting in a bulk secretion of multiple factors. However, pro- and antiangiogenic regulators have been shown to organize into separate, distinct α-granules in resting platelets.7 Then some of these factors could be selectively released under various platelet stimulations. Activation of human platelets with adenosine diphosphate (ADP) stimulates the release of VEGF, but not endostatin, promoting both migration of human umbilical vein endothelial cells and formation of capillary structures. Conversely, thromboxan-A2–stimulated platelet drives endostatin secretion, but not VEGF, which in turn inhibits cell migration and capillary-like tube formation.8 Activation of specific cell surface receptors might define the role of platelets in controlling the angiogenic switch, as shown by the use of protease-activated receptor 1 (PAR1)- and PAR4-activating peptide (PAR1-activating peptide, PAR4-activating peptide), ADP acting on P2Y1/P2Y12, and glycoprotein VI-targeting collagen-related peptide that triggered differential release of pro- and antiangiogenic proteins.9,10 Despite this in vitro evidence, better characterization of pathophysiologic situations leading to selectivity of agonist productions is required.
Despite the impact of platelets in tumor growth and angiogenesis, since Trousseau’s observation more than a century ago on the association between cancer and venous thromboembolism11 and the seminal work of Gasic in middle 1900s,12 it is well recognized that one of the major roles of platelets in the poor outcome of cancer patients occurs during hematogenous dissemination of circulating tumor cells (CTCs) by contributing to their survival in flux, extravasation, and establishment of metastases.1,2,12
Platelets and survival of CTCs in the bloodstream
Characteristics of the role of platelets in metastasis have been recently very well reviewed.1,2 Here we will report only some principle aspects as an overview for the readers. Because shear stress and natural killer (NK) cytotoxic cells are the main threats to CTCs, cancer cell survival in the bloodstream remains a crucial step for metastasis. Mechanisms by which platelets assist CTCs depend on the ability of platelets to act collectively with coagulation factors to protect CTCs within the bloodstream.1 In this context, it has been clearly described that several factors such as tissue factor (TF), thrombine, or ADP secreted by either platelets or CTCs induce platelet activation and formation of a “platelet–cancer cell aggregate.”13,14 Activated platelets crosslink together through different cell surface receptors interacting with their soluble ligands, forming platelet clots protecting CTCs from shear stress and immune attacks. Using perfusion chambers, Konstantopoulos and colleagues showed that platelet P-selectin interacts with tumor CD44, inducing platelet rolling on CTCs and leading to a weak attachment. Fibrinogen receptor GPIIb-IIIa (αIIbβ3 integrin) is also involved in platelet–CTC emboli by converting this initial weak attachment into a strong and stable interaction.15,16 Beyond their role as a “shield” for tumor cells in the circulation, platelets also exert paracrine suppression of NK-mediated cytolytic activity. Notably, transforming growth factor-β (TGF-β) released from activated platelets counteracts NK granule mobilization, cytotoxicity, and interferon-γ secretion.17 In addition, recent studies have demonstrated that platelet–CTC interaction can also lead to the transfer of platelet major histocompatibility complex class I to tumor cells, thereby preventing NK cell recognition.18
Platelets, CTCs, and epithelial-to-mesenchymal transition
The epithelial-to-mesenchymal transition (EMT) process was primarily described as a key regulatory mechanism of embryonic development, whereby epithelial cells acquire mesenchymal/fibroblast-like properties allowing disruption of cell–cell adherence, loss of apico-basal polarity, matrix remodeling, and increased motility and invasiveness.19 EMT is also required for cancer cell metastasis by increasing the capacity of cell invasion and access to the vasculature and by conferring tumor cell resistance to apoptosis and senescence.20 EMT is promoted by the tumor microenvironment including inflammation, immune, and stromal cells, as well as hypoxia and the component of the extracellular matrix.21 Interestingly, CTCs isolated from peripheral blood of breast and prostate cancer patients mostly coexpress epithelial and mesenchymal markers, including E-cadherin, cytokeratin, epithelial cell adhesion molecule, vimentin, and N-cadherin.22 Although previous models of tumor progression proposed that the metastatic potential of tumor cells was entirely acquired during EMT at the primary site, molecular mechanisms by which CTCs maintain an EMT state remain unclear. In this context, recent studies highlighted that EMT-like events occur during the intravascular transit of CTCs with a potential major contribution of platelets by secreting EMT inducers. Consistent with this hypothesis, it has been shown that CTC clusters isolated from patients with advanced breast cancers highly exhibit mesenchymal markers and show an abundance of attached CD61-positive/platelets.23 Labelle and colleagues showed that pretreatment of MC38 colon and Ep5 breast carcinoma cells with platelets promoted lung metastasis by increasing tumor cell seeding and inducing an EMT-like phenotype. In addition, platelet pretreatments increased expression of prometastatic genes and activation of the TGF-β/Smad signaling pathway in these cells. Platelets isolated from Pf4-cre+;TGF-β1fl/fl mice, which lack TGF-β1 synthesis specifically in MKs and platelets, did not promote tumor cell metastasis in wild-type (WT) animals, demonstrating the role of platelet-derived TGF-β in promoting lung metastasis. However, cells treated only with platelet releasate did not undergo EMT, indicating that activation of other pathways was required. By using breast carcinoma cells expressing a nonfunctional mutant of nuclear factor (NF)-κB, the authors demonstrated that the EMT program was also dependent on activation of the NF-κB pathway, specifically triggered after direct contact between tumor cells and platelets. In summary, platelet–tumor cell physical interaction and secretion of TGF-β1 from platelet α-granules synergize to promote an EMT-like transition and metastasis24 (Figure 1).
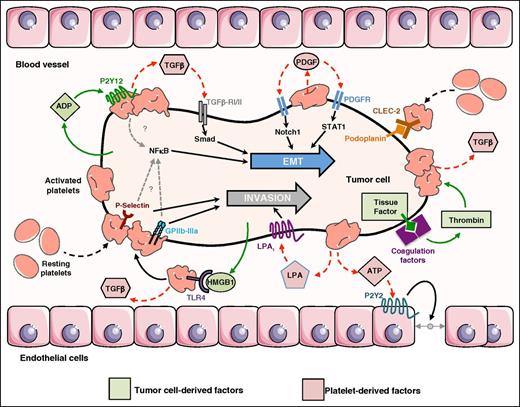
Role of platelets in the control of EMT and invasion of CTCs. CTC-mediated platelet activation depends on platelet/tumor cell interaction involving the nonrestricted list of platelet adhesive molecule P-selectin, GPIIb-IIIa (αIIbβ3 integrin),41 and interaction of C-type lectin-like receptor (CLEC)-2 with tumor-derived podoplanin.45 Tumor cells secrete ADP that interacts with P2Y12, high-mobility group box1 protein 1 (HMGB1) that interacts with TLR-4 and express the tissue factor that activates the coagulation factors (VIIa/Xa) leading to thrombin production. All these factors mediate platelet activation and secretion of TGF-b.30,32,49 Then, TGF-β activates the TGF-β–RI/II–Smad signaling pathway that combines with an unidentified platelet adhesion-dependent mechanism leading to NF-κB activation, which induces EMT on CTCs.24 Released PDGF can activate Notch1 and STAT1 signaling pathways, inducing and/or maintaining EMT on CTCs.25,26 Direct interaction of platelet membranes exposes active P-selectin and GPIIb-IIIa,41 and the secretion of LPA by activated platelets supports CTC invasion39,40 and ATP promotes transendothelial cell migration.37 Figure was generated using the database of images from Servier Medical Art from Servier (http://creativecommons.org/licenses/by/3.0/fr/).
Role of platelets in the control of EMT and invasion of CTCs. CTC-mediated platelet activation depends on platelet/tumor cell interaction involving the nonrestricted list of platelet adhesive molecule P-selectin, GPIIb-IIIa (αIIbβ3 integrin),41 and interaction of C-type lectin-like receptor (CLEC)-2 with tumor-derived podoplanin.45 Tumor cells secrete ADP that interacts with P2Y12, high-mobility group box1 protein 1 (HMGB1) that interacts with TLR-4 and express the tissue factor that activates the coagulation factors (VIIa/Xa) leading to thrombin production. All these factors mediate platelet activation and secretion of TGF-b.30,32,49 Then, TGF-β activates the TGF-β–RI/II–Smad signaling pathway that combines with an unidentified platelet adhesion-dependent mechanism leading to NF-κB activation, which induces EMT on CTCs.24 Released PDGF can activate Notch1 and STAT1 signaling pathways, inducing and/or maintaining EMT on CTCs.25,26 Direct interaction of platelet membranes exposes active P-selectin and GPIIb-IIIa,41 and the secretion of LPA by activated platelets supports CTC invasion39,40 and ATP promotes transendothelial cell migration.37 Figure was generated using the database of images from Servier Medical Art from Servier (http://creativecommons.org/licenses/by/3.0/fr/).
In addition to TGF-β, other factors released by activated platelets may play supplementary roles in promoting EMT in CTCs. Among them, PDGF-D was shown to increase the expression of Notch-1 in cancer cells, which is known to be involved in a conserved ligand receptor pathway and as an inducer of EMT.25 Paracrine activation of platelet-derived growth factor receptor signaling through signal transducer and activator of transcription (STAT)1 may also contribute to the maintenance of EMT26 (Figure 1).
Recent studies identified new tumor-derived factors that act on platelets to promote platelet-induced EMT. TF is a transmembrane protein that regulates extrinsic coagulation.27 Its overexpression in several cancers correlates with an increased risk of metastasis and decreased overall survival.28,29 The treatment of ovarian cancer cells isolated from ascitic fluid of patients with human platelets promoted the formation of spheres and showed a reduction in the epithelial marker E-cadherin and an increase in both CD44 stem cell marker and expression of TF.30 The platelet-induced phenotypic changes in patient-derived ovarian cancer cells was associated with an increase in cell migration and TF activity, supporting the potential role of TF in retaining an EMT state of CTCs, thereby promoting cancer cell metastasis.30 Activation of different types of platelet cell surface receptors may also support the induction and/or conservation of EMT in cancer cells. P2Y12 is an ADP receptor playing a crucial role in platelet activation, granule secretion, and thrombus formation.31 A lack of this receptor diminished the ability of Lewis Lung carcinoma cells to induce platelet shape change, aggregation, and the release of active TGF-β1. Incubation of cancer cells with platelets lacking P2Y12 also decreased platelet-induced invasiveness and EMT-like transformation, and P2Y12-deficient mice exhibit a significant decrease in pulmonary metastasis.32 Therefore, released ADP acting on platelet P2Y12 induces EMT, invasion, and metastasis in cancer cells, notably by controlling the release of TGF-β132 (Figure 1).
Platelets and CTC invasion/extravasation
Beyond their role in inducing and/or maintaining EMT-like phenotype in CTCs, platelets also support CTC extravasation to secondary sites. Following Felding Habermann’s works demonstrating the role of β3 integrin family members (platelet αIIbβ3, tumor αVβ3) in CTC adhesion and invasion under flow conditions,33-35 recent studies revealed that low shear stress upregulated matrix metaloproteinase 2 (MMP-2), MMP-9, VEGF, and αVβ3 integrin, promoting MDA-MB-231 breast carcinoma cell adhesion and invasion, as well as mobilizing both phosphatidylinositol 3-kinase/Akt signaling pathways and NF-κB activation.36 Using Munc13-4–deficient mice, which present a defect in dense granule secretion but not in α-granule release, Schumacher and colleagues demonstrated that tumor cell–induced platelet aggregation leads to the release of adenosine triphosphate (ATP) stored in dense granules. Then, released ATP binds to the endothelial purinergic P2Y2 receptor, stimulating cancer cell intravasation and metastatic dissemination to the lung, thus demonstrating that ATP secreted from platelets supports platelet-induced tumor cell transendothelial migration and metastatic dissemination37 (Figure 1). Coincubation of platelets with metastatic breast cancer cells under both steering38 and passive39 conditions mediate lysophosphatidic acid (LPA) production that subsequently activates tumor LPA1 receptor promoting cell invasion via a PI3K/zinc finger E-box–binding homeobox 1/microRNA-21–dependent signaling pathway.40
The physical interaction between platelets and tumor cells is a well-known prerequisite for platelet prometastatic activity mediating secretion of protumoral factors. However, secretion of paracrine components active on tumor cells might not be the sole mechanism in platelet-promoting metastasis. Pang and colleagues used a series of cancer cell lines from different origins (MM170 melanoma, MOLT-4T lymphoma, and MDA-MB-231 breast cancer) that once incubated in vitro with washed thrombin-activated platelet membranes exhibited a systematic increase in aggressiveness in a Matrigel-degrading assay.41 Dual blocking of the platelet-membrane proteins P-selectin and GPIIb-IIIa prevented interactions between platelet membranes and tumor cells and significantly reduced the ability of cancer cells to degrade Matrigel, confirming the role of both receptors in assisting tumor cells in the extravasation process.41 In addition, such incubation of tumor cells with washed thrombin-activated platelet membranes prior to injection in the mouse lateral tail vein lead to a significant increase in lung metastasis formation, confirming that priming tumor cells with platelet membranes lead to sustained modifications promoting cancer aggressiveness and metastasis independently of platelet-secreted factors (Figure 1).
Watson’s group identified the C-type lectin receptor CLEC-2 in platelets that, once activated by the snake venom rhodocytin, induces platelet activation through CLEC-2’s cytosolic domain binding of Syk and downstream activation of phospholipase Cγ2.42 podoplanin, a marker of lymphatic vessels, has also been reported in cancer cells, contributing to cancer pathogenesis by promoting tumor cell invasion and spreading.43 Intriguingly, interaction of podoplanin with CLEC-2 controls tumor cell–induced platelet aggregation and metastasis.44,45 Recent development of specific blockers of the binding domain of CLEC-2 that interacts with podoplanin revealed a promising therapeutic potential for blocking platelet-induced metastasis46,47 (Figure 1).
Lipopolysaccharide was shown to induce thrombocytopenia by involving stimulation of platelet degranulation and potentiation of platelet aggregation via activation of a Toll-like receptor 4 (TLR4)/myeloid differentiation factor 88/cycling guanosine monophosphate-dependent pathway.48 Pretreatments of WT animals with lipopolysaccharide 6 hours before injection of B16F10 melanoma and LCC lung carcinoma cells significantly increased lung and liver metastasis formation, which was markedly weak in TLR4−/− mice.49 Antiplatelet agents (clopidogrel, aspirin) alone or in combination drastically inhibited lung metastasis of B16F10 cells in WT animals but had no impact on the basal lower number of lung metastasis foci in TLR4−/− mice. Furthermore, TLR4-null platelets were impaired in tumor cell interaction and neutralization of HMGB1 using an anti-HMGB1 monoclonal antibody, which partially blocked WT platelet adhesion on tumor cells and release TGF-β in vitro, and decreased by 50% the formation of lung metastases in vivo in a TLR4-dependent manner.49 Therefore, platelets promote tumor metastasis via interaction between TLR4 and tumor cell-released HMGB1 (Figure 1).
Platelets and bone metastasis
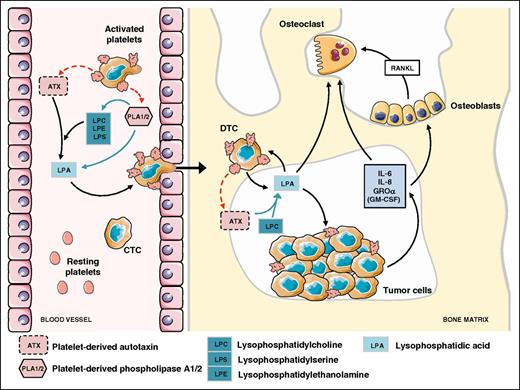
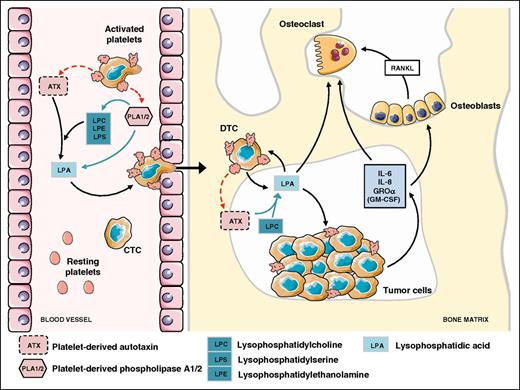
Bone is a sanctuary for metastatic cells.50 Solid tumors frequently develop bone tropism properties as observed in breast, prostate, lung, thyroid, and renal cancers.50,51 Once seeded at the bone site, metastatic cells interfere with the bone remodeling process by promoting either osteolytic lesions (bone destruction) or osteosclerotic metastases (excessive bone formation). It is now well established that platelets are essential for cancer metastasis dissemination and progression to the bone. Bakewell and colleagues showed that β3 integrin-deficient mice exhibited a 95% decrease in skeletal tumor burden after intracardiac inoculation of B16 melanoma cells.52 By using osteoclast defective src−/− mice and specific platelet aggregation inhibitors, the authors could discriminate the roles of β3 integrins of osteoclasts and of platelets. Integrin αIIbβ3 controls both platelet aggregation and CTC homing to the bone microenvironment, whereas osteoclast integrin αVβ3 drives bone osteolysis.52 Our group extended the role of platelet αIIbβ3 integrin, not only to the onset of skeletal metastasis but also to the progression of bone metastasis, by treating metastasis harboring mice with integrilin, a pharmacologic αIIbβ3 antagonist.38 This study also revealed the role of a platelet-derived bioactive lipid, LPA, as an enhancer of skeletal metastasis.38 In a specific mouse regiment setting designed not to interfere with the role of platelets in the bone homing of CTCs, integrelin treatment was initiated only after previous establishment of bone metastases. Interestingly, treated animals exhibited thrombocytopenia associated with a significant reduction in plasma levels of LPA and a decrease in the extent of osteolytic bone lesions.38 The role LPA in bone metastasis was further demonstrated due to its capacity of promoting tumor cell proliferation, survival, migration, and bone resorption through both an indirect action mediating cancer cell secretion of pro-osteoclastic cytokines (interleukin [IL]-6, IL-8, monocyte chemoattractant protein [MCP]-1, Groα) and a direct stimulation of osteoclast differentiation and bone resorption activity38,53,54 (Figure 2). More recently, underlying mechanisms controlling platelet-derived LPA production on cancer cell stimulation has been partly elucidated. Autotaxin (ATX), through its lysophospholipase D activity, is known to control physiologic levels of LPA in the blood circulation.55 We demonstrated that circulating nontumoral ATX can be stored in α-granules of resting platelets and released on breast cancer cell stimulation.39 In vitro cell adhesion and proliferation assays indicated that exogenous ATX could bind both tumor αVβ3 and platelet αIIbβ3 integrins.39,56,57 Such a physical interaction between ATX and β3 integrins might promote local production of LPA, which in turn could act more efficiently on cancer cells, stimulating metastasis and cancer dissemination to the bone39 (Figure 2).
Role of nontumoral ATX derived from platelet α-granules in LPA production promoting bone metastasis of breast cancer cells. Interaction of CTCs with platelets induces the generation of LPA through the secretion of LPA and LPA precursors involving phospholipase A1/A2 activities and lysopholipase D activity of ATX released from α-granules.39 Then, LPA promotes transendothelial cell migration and establishment of a disseminated tumor cell into the bone marrow.81 LPA enhances tumor cell proliferation and induces bone resorption through a direct action on osteoclasts54 and indirectly throughout the secretion of anti-(GM-CSF) and pro-osteoclastic factors (IL-6, IL-8, GROα) that also act directly on osteoclasts or indirectly on osteoblasts by upregulating the release of the pro-osteoclastic factor RANKL.38,53 Figure was generated using the database of images from Servier Medical Art from Servier (http://creativecommons.org/licenses/by/3.0/fr/).
Role of nontumoral ATX derived from platelet α-granules in LPA production promoting bone metastasis of breast cancer cells. Interaction of CTCs with platelets induces the generation of LPA through the secretion of LPA and LPA precursors involving phospholipase A1/A2 activities and lysopholipase D activity of ATX released from α-granules.39 Then, LPA promotes transendothelial cell migration and establishment of a disseminated tumor cell into the bone marrow.81 LPA enhances tumor cell proliferation and induces bone resorption through a direct action on osteoclasts54 and indirectly throughout the secretion of anti-(GM-CSF) and pro-osteoclastic factors (IL-6, IL-8, GROα) that also act directly on osteoclasts or indirectly on osteoblasts by upregulating the release of the pro-osteoclastic factor RANKL.38,53 Figure was generated using the database of images from Servier Medical Art from Servier (http://creativecommons.org/licenses/by/3.0/fr/).
Several antiplatelet therapies have been evaluated in the context of bone metastasis. Weilbaecher and colleagues investigated the effect of the soluble ADPase APT102 administered in combination with an inhibitor of TXA2 synthesis (acetylsalicylic acid/aspirin).58 Although ATP102 and aspirin treatment did not affect primary tumor growth, the combined treatment significantly attenuated the extent of melanoma and breast cancer bone metastasis in mice.58
MKs and bone metastasis
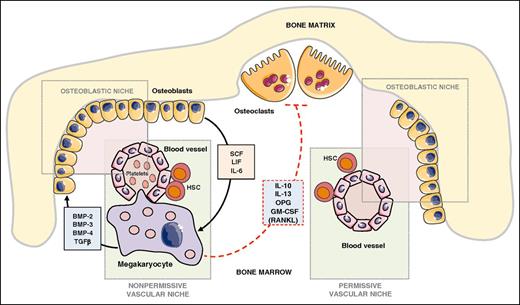
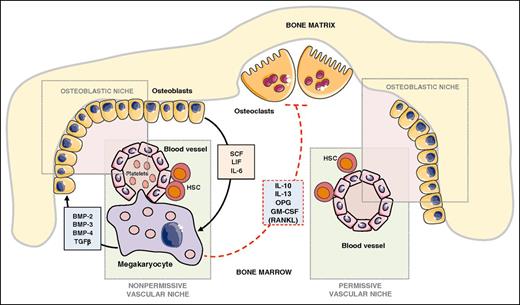
The bone remodeling process, which is tuned by the bone formation activity of osteoblasts and the bone resorption function of osteoclasts, is known for contributing to the attraction of circulating bone seeking tumor cells.59,60 MKs are powerful modulators of bone mass.61 Transcription factors Nf-E2 and GATA-1 are required for platelet production and MK terminal differentiation, as revealed by induction of thrombocytopenia in knockout mice for either Nf-E2 or GATA-1.62 In addition, Nf-E2−/− and GATA-1−/− mice exhibit a high bone mass phenotype.61 MKs produce a large variety of bone formation promoting factors such as bone morphogenetic protein (BMP)-2, -4, and -6 and TGF-β.63 Meanwhile, osteoblasts secrete a series of cytokines that control hematopoiesis such as LIF, IL-6, and stem cell factor and enhance MK proliferation and differentiation.64 In contrast, MKs inhibit osteoclast differentiation and bone resorption through as yet not unraveled mechanisms because MKs secrete both the activator of osteoclast differentiation (RANKL) and strong inhibitors of osteoclastogenesis and bone resorption (osteoprotegerin, IL-10, IL-13, granulocyte-macrophage colony-stimulating factor [GM-CSF])65 (Figure 3). Thus, the positive action of MKs on bone mass acquisition is the net result of direct and indirect actions of MKs on bone cells. Thrombopoietin (TPO) is a master regulator of magakaryocytopoiesis and platelet production.66 Increased expansion of MKs in Balb/c nude mice in response to TPO prior to intracardiac injection of PC3 prostate cancer cells remarkably decreased the extent of skeletal lesions and metastatic tumor burden.67 This result was rather surprising because an increase in the number of circulating platelets in response to TPO would create a more propitious metastatic environment for PC3 cells in the blood circulation. The platelet production–independent function of MKs might prevail in this phenomenon. In vitro cocultures of K562 human MK precursors or mouse primary MKs derived from bone marrow hematopoietic precursors inhibit the proliferation of prostate carcinoma cell lines (PC3, C4-2b, VCaP).67 Intercellular contacts between MKs and PC3 cells downregulate cyclinD1 and upregulate the proapoptotic factors apoptosis-associated speck-like protein containing a caspase recruitment domain and death-associated protein kinase 1, favoring apoptosis and inhibition of skeletal tumor growth and thereby demonstrating a direct antitumoral action of MKs. As a consequence, the cumulative actions of MKs on tumor cells, osteoblasts, and osteoclasts are likely the reason for inhibition of PC3 cell skeletal metastases.67 Intravenous injection of B16F10 melanoma cells in Nf-E2−/− mice resulted in a strong decrease in lung metastasis formation compared with that obtained in WT animals.16 This report was one of the best confirmations for the major role of platelets in hematologic dissemination of tumor cells. B16F10 cells metastasize to bone after intracardiac injection. One would speculate that such an injection of B16F10 cells in Nf-E2−/− mice would result in a decrease in skeletal metastasis formation, but formal experimental evidence is required. In their study, Li and colleagues showed that, as opposed to their action on prostate cancer cell lines, MKs do not inhibit but instead stimulate the proliferation of osteosarcoma cells.67 Therefore, generalization of MKs as anti-bone metastasis cells of solid tumors requires more support.
Role of MKs in bone remodeling and metastatic niches. Functional osteoblastic and vascular/hematopoietic niches are required for successful establishment of bone metastases.74 MKs and hematopoietic stem cells are located in the vascular niche. MKs control bone mass through the secretion of bone formation factors (BMP-2, −3, −4, TGF-β) and production of mostly inhibitors of osteoclast function (OPG, IL-10, IL-13, GM-CSF).65 In turn, osteoblasts secrete a series of cytokines that control hematopoiesis (stem cell factor, LIF, IL-6) and MK proliferation and differentiation.64 In a bone metastasis animal model, MKs inhibited skeletal destruction and induced apoptosis of prostate cancer cells,67 suggesting that the presence or absence of MKs might convert the vascular/osteoblastic niche from a nonpermissive to a permissive environment for successful establishment of metastases. Figure was generated using the database of images from Servier Medical Art from Servier (http://creativecommons.org/licenses/by/3.0/fr/).
Role of MKs in bone remodeling and metastatic niches. Functional osteoblastic and vascular/hematopoietic niches are required for successful establishment of bone metastases.74 MKs and hematopoietic stem cells are located in the vascular niche. MKs control bone mass through the secretion of bone formation factors (BMP-2, −3, −4, TGF-β) and production of mostly inhibitors of osteoclast function (OPG, IL-10, IL-13, GM-CSF).65 In turn, osteoblasts secrete a series of cytokines that control hematopoiesis (stem cell factor, LIF, IL-6) and MK proliferation and differentiation.64 In a bone metastasis animal model, MKs inhibited skeletal destruction and induced apoptosis of prostate cancer cells,67 suggesting that the presence or absence of MKs might convert the vascular/osteoblastic niche from a nonpermissive to a permissive environment for successful establishment of metastases. Figure was generated using the database of images from Servier Medical Art from Servier (http://creativecommons.org/licenses/by/3.0/fr/).
Suva and colleagues developed a mouse model of platelet-type von Willebrand disease (Pt-vWD) that corresponds to a human bleeding disorder due to a point mutation in GPIbα. Pt-vWD is characterized by a higher affinity of platelets to the soluble form of von Willebrand factor, leading to a persistent thrombocytopenia.68 Pt-vWD animals exhibit an increased number of MKs in the bone marrow and a high bone mass phenotype.68 Due to its specific expression in MKs and platelets, GPIbα could become an attractive target for the development of new skeletal metastasis therapies. However, controversies have been raised in the past for the role of GPIbα in tumor cell–induced platelet aggregation, with some reports showing its positive contribution,69 whereas others described no impact of blocking GPIbα antibodies.70 Also, in an experimental metastasis model using B16F10 melanoma cells, mice lacking GPIbα developed a lower number of lung metastases than WT mice,71 whereas functional inhibition of GPIbα using monoclonal antibodies in vivo led to a strong increase in pulmonary metastasis.72 Such discrepancies are still not well understood. Nevertheless, due to their specific location in the vascular niche, MKs are very tempting targets for blocking the formation of skeletal metastases because they might be the first cells encountered by metastatic cancer cells during extravasation at early steps of bone colonization.
MKs and the vascular/hematopoietic niche
According to the “seed and soil” theory presented by Sir James Paget in 1889,73 vascular and osteoblastic niches are now considered as structural microenvironments required for establishing bone metastases.74 Manipulating the hematopoietic niche using anti-SDF1/CXCR4 strategies successfully block skeletal metastases75 and alteration of the osteoblastic niche using zoledronic acid markedly affect bone metastasis formation.76 The recent work of Méndez-Ferrer and colleagues on nestin-positive cells showed that both vascular and osteoblastic niches might overlap anatomically, forming a unique entity.77 Nonetheless, intravital video microscopy experiments performed by Kienast and colleagues revealed that a strict perivascular location is required for survival of tumor cells at this step of the metastasis cascade.78 The contribution of MKs in malignancy and bone marrow vascular niches has been recently nicely reviewed elsewhere.79 However, as shown by Li and colleagues, expansion of MKs in the vascular niche in the bone marrow of mice treated with TPO inhibits bone metastasis formation.67 Altogether, these observations suggest that absence or presence of MKs in specialized vascular niches might convert those niches from a permissive to a nonpermissive environment for metastasis establishment. In other words, MK activity might contribute to the seed and soil theory73 for successful metastasis that require suitability between CTCs and target tissue, which here is bone. Interestingly, in their recent review, Psaila and colleagues hypothesized the potential existence of specialized subpockets of the hematopoietic microenvironment modulated by osteoblast- and endothelial-derived factors controlling stem cell behavior.
Conclusion
Tail vein and intracardiac injections of tumor cells have been extensively used in metastasis studies; however, these types of injections model only certain aspects of the metastasis process. The use of animal models based on orthotopic injection of tumor cells allowing the formation of spontaneous metastasis would provide, in the future, better understanding of the role of platelets and MKs in early stages of metastasis development. Nevertheless, recent in vitro and in vivo findings made a great advance in our knowledge of how platelets contribute to metastatic dissemination of cancer cells, which may support the development of new antimetastasis therapies. Intriguingly, as opposed to platelets that promote metastasis, the recent identification of the negative action of MKs in bone metastasis formation revealed that a global effect of the MK–platelet axis could not be considered pro- or antimetastatic. Acting on platelets directly might be beneficial to patients by affecting metastasis without any specification for the secondary site seeding of tumor cells (lungs, bone, etc). It is unlikely that acting on MKs would offer the same possibility, but targeting bone marrow–specialized niches through MK activity may provide new therapeutic opportunities. However, further studies are required to validate such contentions, especially through manipulating MKs in vivo that may encounter adverse effects. As an example, using TPO in preventing metastasis may not be an appropriate strategy in the clinic because of an immediate increase in the number of circulating platelets that would favor platelet/tumor cell interactions, thereby increasing the risk of venous thromboembolism.80 Nevertheless, such therapeutic strategies could be associated with other antiplatelet therapies. More precisely, a new area may be opened in the development of drugs that specifically target the prometastatic activity of platelets such as CLEC-2/podoplanin,45 TLR-4/HMGB1,49 or ATX activity39 without affecting their physiologic functions in hemostasis.
Acknowledgments
This review was supported by grants from the INSERM, the University of Lyon (O.P.), the Comité Départemental de la Loire de la Ligue Contre le Cancer (O.P.), and the French Fondation pour la Recherche sur le Cancer, Association pour la Recherche sur le Cancer (ARC) (O.P.). R.L. is a recipient of a grant fellowship from the Fondation ARC.
Authorship
Contribution: R.L. and O.P. wrote the review and designed the figures and tables.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Olivier Peyruchaud, INSERM U1033, Faculté de Médecine Lyon Est, Rue Guillaume Paradin, 69372 Lyon cedex 08, France; e-mail: olivier.peyruchaud@inserm.