To the editor:
Ibrutinib is an irreversible inhibitor of Bruton tyrosine kinase in the B-cell receptor signaling pathway. In randomized trials, ibrutinib is effective as first-line treatment of chronic lymphocytic leukemia (CLL)/small lymphocytic lymphoma (SLL) (compared with chlorambucil),1 for relapsed/refractory CLL/SLL (compared with ofatumumab or combined with bendamustine/rituximab),2,3 or for relapsed/refractory mantle cell lymphoma (compared with temsiroliums),4 with promising results in the treatment of Waldenström macroglobulinemia.5 It is anticipated that ibrutinib will become an important part of the therapeutic armamentarium for these conditions. Randomized trials suggest that ibrutinib may increase the risk of atrial fibrillation (AF) as compared with chlorambucil1 or ofatumumab.2 In the general population, AF is strongly associated with heart failure and arterial thromboembolism, which result in substantial morbidity and mortality. There is uncertainty over the magnitude of the increase in AF risk attributable to ibrutinib because the absolute numbers of incident AF cases are small in individual studies.
We undertook a systematic review and meta-analysis to (1) estimate the magnitude of the increase in AF risk among ibrutinib recipients, as compared with alternative therapies and (2) quantify the frequency of AF reported among ibrutinib recipients. We searched MEDLINE and EMBASE, and proceedings from the American Society of Hematology, the European Haematology Association, and the American Society of Clinical Oncology for articles describing AF rates in recipients of ibrutinib. The following search terms were used: ibrutinib; imbruvica; or PCI-32765. Animal studies, case reports, case series (ie, that reported on consecutive AF cases), cross-sectional studies, editorials, phase I/dose-finding studies, and conference abstracts more than 12 months old were excluded.
Two independent reviewers screened the articles’ titles and abstracts for eligibility. Cases of disagreement were resolved by a third reviewer. Papers identified after title and abstract screening were obtained in full. When data from the same cohort of participants were presented in different papers, only the manuscript with the larger sample size was included in the meta-analysis. The following data were extracted from eligible full text manuscripts: design, disease, sample size, treatments, participant age and sex, follow-up duration, AF rates, and where reported, AF ascertainment strategies, past history of cardiovascular disease, AF, or hypertension.
Statistical analysis was performed using STATA 14 (StataCorp, College Station, TX). To evaluate the increase in the risk of incident AF, the primary meta-analytic approach was a fixed effects model using the Mantel-Haenszel method. A sensitivity analysis was performed using a DerSimonian and Laird random effects model. Heterogeneity of studies was evaluated by Cochran’s Q and the I2 statistic. Pooled AF rates were estimated as follows: we multiplied the median follow-up duration by the sample size. Crude study-specific AF rates were then calculated by dividing the number of incident AF cases by the total number of person-months follow-up. AF rates were then pooled using the “metaprop” command in STATA, which computes the pooled estimates after the Freeman-Tukey double arcsine transformation to stabilize the variances.
The search strategy yielded 1871 unique abstracts. Thirty-nine full text manuscripts and 44 conference abstracts were reviewed. Of these 83 papers, 63 were excluded because they lacked data on the occurrence of AF (n = 45), did not study ibrutinib (n = 2), were dose-finding studies (n = 1) or case series (n = 1), or included data that were presented in another included manuscript (n = 14). Thus, 20 manuscripts, reporting on the occurrence of AF in individuals treated with ibrutinib, contributed to the meta-analysis (Table 1).6-21 Four of these studies were randomized controlled trials, 10 phase II studies, one prospective cohort study, and 5 retrospective cohort studies. In total, 14 studies included CLL/SLL patients; 5 studies included mantle cell lymphoma patients; 2 studies included Waldenström macroglobulinemia patients, and one study included follicular lymphoma patients.
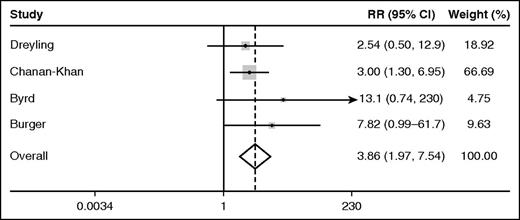
Among the 4 randomized trials of ibrutinib, the pooled relative risk (95% confidence interval, CI) of AF associated with ibrutinib as compared with the comparator was 3.9 (2.0-7.5, P < .0001) according to the fixed effects model (Figure 1). By the random effects model, the pooled relative risk (95% CI) of AF in ibrutinib recipients was 3.5 (1.8-6.9, P < .0001). The I2 statistic for heterogeneity was 0%, and the heterogeneity  was 1.74 (P = .6), suggesting homogeneity of results among the randomized trials. Over median follow-ups of up to 26 months, the pooled rate (95% CI) of AF among ibrutinib recipients among all 20 studies cited was 3.3 (2.5-4.1) per 100 person-years. The pooled rate (95% CI) of AF among participants receiving the nonibrutinib therapy in the 4 randomized trials included was 0.84 (0.32-1.6) per 100 person-years.
was 1.74 (P = .6), suggesting homogeneity of results among the randomized trials. Over median follow-ups of up to 26 months, the pooled rate (95% CI) of AF among ibrutinib recipients among all 20 studies cited was 3.3 (2.5-4.1) per 100 person-years. The pooled rate (95% CI) of AF among participants receiving the nonibrutinib therapy in the 4 randomized trials included was 0.84 (0.32-1.6) per 100 person-years.
Forest plot of the fixed effects relative risk (RR) of AF in ibrutinib recipients as compared with alternative therapy.
Forest plot of the fixed effects relative risk (RR) of AF in ibrutinib recipients as compared with alternative therapy.
This analysis suggests that ibrutinib consistently increases the risk of incident AF compared with alternative therapies. The incidence rates of AF observed in the general adult population have been previously described. Among 7983 community-dwelling adults aged 60-64 years screened by electrocardiogram twice during a mean 6.9 years, the incidence (95% CI) of AF was 0.55 (0.42-0.71) per 100 person-years.22 In the Framingham study, AF incidence was measured by annual questionnaires, by annual electrocardiograms, and from hospital records. The incidence of AF among men aged 65-74 years was 1.8 per 100 person-years, and among women aged 65-74 years was 1.0 per 100 person-years.23 Thus the rate of AF among ibrutinib recipients is substantially higher than the incidence rate observed among the general population. The mechanism(s) by which ibrutinib may promote AF are unknown. Although Bruton tyrosine kinase and tec protein kinases, which are inhibited by ibrutinib, are expressed in cardiac tissue,24,25 further research is needed to elucidate specific molecular pathways.
A number of caveats should be acknowledged in interpreting these data. No study reported methods for identifying AF cases. Because allocation was masked in only one of the randomized trials of ibrutinib,3 bias in the detection of AF between the ibrutinib and comparator arms may have occurred. Furthermore, in populations at high risk for AF (but without known AF), the yield of AF increases with the intensity of surveillance. Therefore, if screening for AF was not conducted in an intensive or systematic manner, the rates of AF reported in the present meta-analysis may underestimate the true rates. The baseline prevalence of AF was reported in only 2 studies in this meta-analysis. We cannot exclude the possibility that the increase in the risk of incident AF observed in the ibrutinib arms of the randomized trials is simply an unmasking of preexisting AF. Given the limited duration of follow-up in studies to date, it is uncertain whether the rate of AF will remain constant over time, or whether it will reduce or increase with prolonged ibrutinib use.
Patients treated with ibrutinib should be closely monitored for the development of AF, which is associated with a 5-fold increased risk of ischemic stroke, a leading cause of morbidity and mortality, in the general population. Further research is necessary to determine the frequency with which AF affects ibrutinib recipients, the temporal relationship between duration of ibrutinib exposure and AF occurrence, predictors of AF among ibrutinib recipients, the thromboembolic risk in ibrutinib recipients who develop AF (especially given the antiplatelet effect of ibrutinib), and how they should optimally be managed.
Authorship
Acknowledgments: D.P.L. is supported by the E. J. Moran Campbell Career Award from McMaster University. C.H. is supported by a fellowship from the Department of Oncology, McMaster University. D.S. is supported by a fellowship award from the Canadian Institutes of Health Research.
Contribution: D.P.L. conceived the study idea, designed the study, collected data, performed statistical analysis, and drafted the manuscript. F.C. designed the study, collected data, and revised the manuscript. C.H. designed the study and revised the manuscript. A.D. collected data. J.S.H. interpreted the data and revised the manuscript. G.F. interpreted the data and revised the manuscript. D.S. designed the study, collected data, and revised the manuscript.
Conflict-of-interest disclosure: D.L. has received honoraria from Janssen Pharmaceuticals. G.F. has received honoraria and research funding from Janssen Pharmaceuticals. All other authors declare no competing financial interests.
Correspondence: Darryl P. Leong, C3-106, David Braley Building, Hamilton General Hospital, 237 Barton St East, Hamilton, ON L8L 2X2, Canada; e-mail: leongd@phri.ca.
References
Author notes
D.P.L. and F.C. contributed equally to this study.