In this issue of Blood, O’Regan et al have extended our understanding of von Willebrand factor (VWF) in the pathogenesis of malaria.1 According to the World Health Organization (http://www.who.int/gho/malaria/en/), malaria affects 3.2 billion people in 97 countries with 198 million cases having occurred in 2013, and of those, 584 000 died. Ninety percent of those deaths in 2013 were children under the age of 5. The most devastating form of the disease is cerebral malaria, which occurs most frequently in young children. Although blood coagulation changes such as disseminated intravascular coagulation have been recognized since the 1970s,2 recent studies have focused on markers of these hemostatic changes as being most prevalent in cerebral malaria caused by Plasmodium falciparum. Cerebral malaria is more lethal in children than adults. Exchange transfusion has been used as an aggressive adjunct therapy for this condition.3
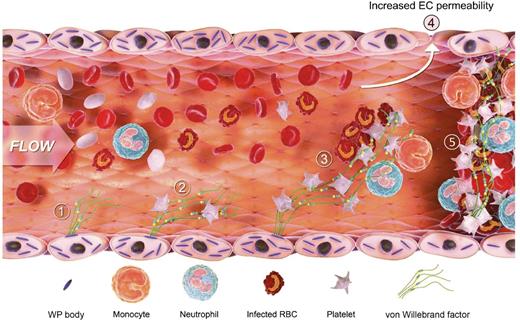
This demonstrates the sequential role of VWF in the process of central nervous system (CNS) malaria. Endothelial cells are activated and release VWF from the Weibel Palade bodies into circulating blood, platelets adhere, parasite-infected red blood cells (RBCs) are recruited, and VWF induces increased endothelial cell permeability and leads to CNS thrombosis. EC, endothelial cell; WP, Weibel Palade. The figure has been adapted from Figure 6 in the article by O’Regan et al that begins on page 1192.
This demonstrates the sequential role of VWF in the process of central nervous system (CNS) malaria. Endothelial cells are activated and release VWF from the Weibel Palade bodies into circulating blood, platelets adhere, parasite-infected red blood cells (RBCs) are recruited, and VWF induces increased endothelial cell permeability and leads to CNS thrombosis. EC, endothelial cell; WP, Weibel Palade. The figure has been adapted from Figure 6 in the article by O’Regan et al that begins on page 1192.
Studies of VWF and its propeptide, VWFpp, demonstrated activation of endothelial cells with release of VWF and VWFpp from the Weibel Palade bodies of endothelial cells into plasma with an increase in the VWFpp/VWF:antigen ratio in patients with cerebral malaria.4 Recently, a donkey antibody has been developed that recognizes the “activated” form of VWF that exists in a conformation that spontaneously binds to platelets.5 Using this approach, activated VWF has been identified in the plasma of patients with cerebral malaria.6 Recently, additional hemostatic abnormalities have been identified including a reduction of VWF-cleaving protease, ADAMTS13,7 in plasma and a loss of endothelial protein C receptor (EPCR) in the cerebral vessels in patients with cerebral malaria.8 One other historical observation is of interest: severe P falciparum cerebral malaria is less common in blood group O individuals,9 and this finding holds up even with more recent genome-wide association studies. In addition, VWF levels are 25% to 30% higher in non–O blood groups,10 which might suggest an association for VWF levels in the pathogenesis of cerebral malaria.
O’Regan et al help clarify whether VWF levels are the “effect” or the “cause” of decreased malaria resistance. The authors developed a model of cerebral malaria in the mouse using Plasmodium berghei ANKA that recapitulates cerebral malaria in humans. When this infection is carried out on the VWF−/− mouse knockout background, clinical experimental cerebral malaria progression is delayed and the survival of these mice is prolonged. Because platelet counts and parasitemia are identical to controls, this suggests VWF is an active participant in cerebral malaria pathogenesis. This effect might partially explain why malaria resistance could be greater in blood group O individuals who have lower VWF levels. As shown (see figure), endothelial cells are activated and (1) release ultralarge VWF multimers that (2) sequester platelets, (3) recruit malaria-infected RBCs, (4) alter endothelial cell permeability, and (5) progress to microvascular thrombosis.
Although it is likely that other mechanisms interplay with this pathogenic pathway, VWF is not just a simple secondary marker of endothelial cell activation (tails), but a potential regulator (heads) of CNS malaria. There will undoubtedly be more to this story in the future.
Conflict-of-interest disclosure: The author declares no competing financial interests.